Crystal structure of Salmonella typhi outer membrane phospholipase (OMPLA) dimer with bound calcium
Perumal, P., Raina, R., Sreeshma, N.S., Arockiasamy, A., Sundarabaalaji, N.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Phospholipase A1 | 257 | Salmonella enterica subsp. enterica serovar Typhi | Mutation(s): 0 Gene Names: pldA, STY3602, t3340 EC: 3.1.1.32 (PDB Primary Data), 3.1.1.4 (PDB Primary Data) Membrane Entity: Yes | 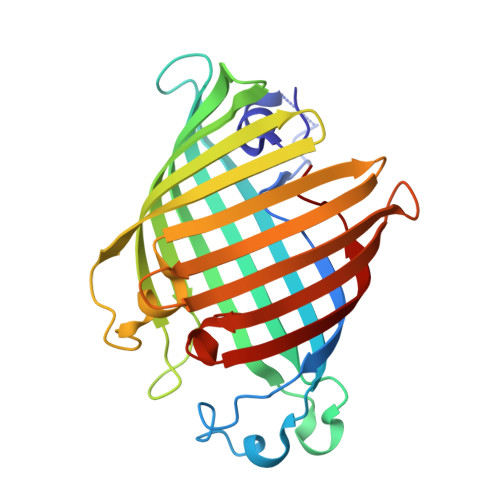 | |
UniProt | |||||
Find proteins for P0A232 (Salmonella typhi) Explore P0A232 Go to UniProtKB: P0A232 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0A232 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| BOG Query on BOG | C [auth A], D [auth A], E [auth A], G [auth A] | octyl beta-D-glucopyranoside C14 H28 O6 HEGSGKPQLMEBJL-RKQHYHRCSA-N |  | ||
| D12 (Subject of Investigation/LOI) Query on D12 | H [auth A], I [auth B] | DODECANE C12 H26 SNRUBQQJIBEYMU-UHFFFAOYSA-N |  | ||
| CA (Subject of Investigation/LOI) Query on CA | F [auth A], J [auth B] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 79.584 | α = 90 |
| b = 83.448 | β = 90 |
| c = 95.644 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| autoPROC | data processing |
| autoPROC | data scaling |
| PHENIX | phasing |
| Coot | model building |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Department of Biotechnology (DBT, India) | India | BT/PR13735/BRB/10/786/2011 and BT/PR28080/BID/7/836/2018. |
| Department of Science & Technology (DST, India) | India | PDF/2016/003347 |
| Department of Biotechnology (DBT, India) | India | BT/PR8636/BRB/10/530/2007 |
| Department of Biotechnology (DBT, India) | India | BT/PR10275/GBD/27/88/2007 |