The Crystal Structure of human MTH1 from Biortus
Wang, F., Cheng, W., Shang, H., Wang, R., Zhang, B., Tian, F.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 7,8-dihydro-8-oxoguanine triphosphatase | 174 | Homo sapiens | Mutation(s): 0 Gene Names: NUDT1, MTH1 EC: 3.6.1.55 (PDB Primary Data), 3.6.1.56 (PDB Primary Data) | 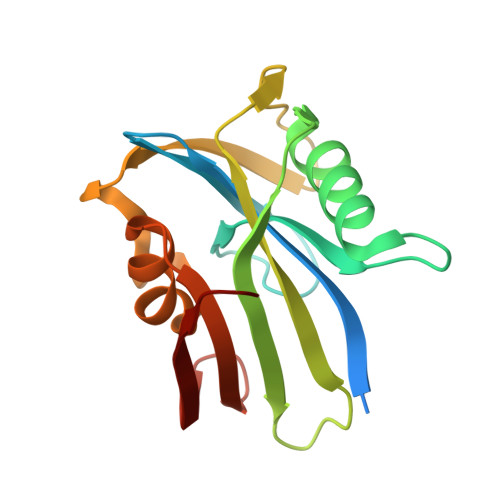 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P36639 (Homo sapiens) Explore P36639 Go to UniProtKB: P36639 | |||||
PHAROS: P36639 GTEx: ENSG00000106268 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P36639 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PG4 Query on PG4 | B [auth A] | TETRAETHYLENE GLYCOL C8 H18 O5 UWHCKJMYHZGTIT-UHFFFAOYSA-N |  | ||
| PEG Query on PEG | C [auth A] | DI(HYDROXYETHYL)ETHER C4 H10 O3 MTHSVFCYNBDYFN-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 36.276 | α = 90 |
| b = 60.533 | β = 90 |
| c = 66.135 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| XDS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |