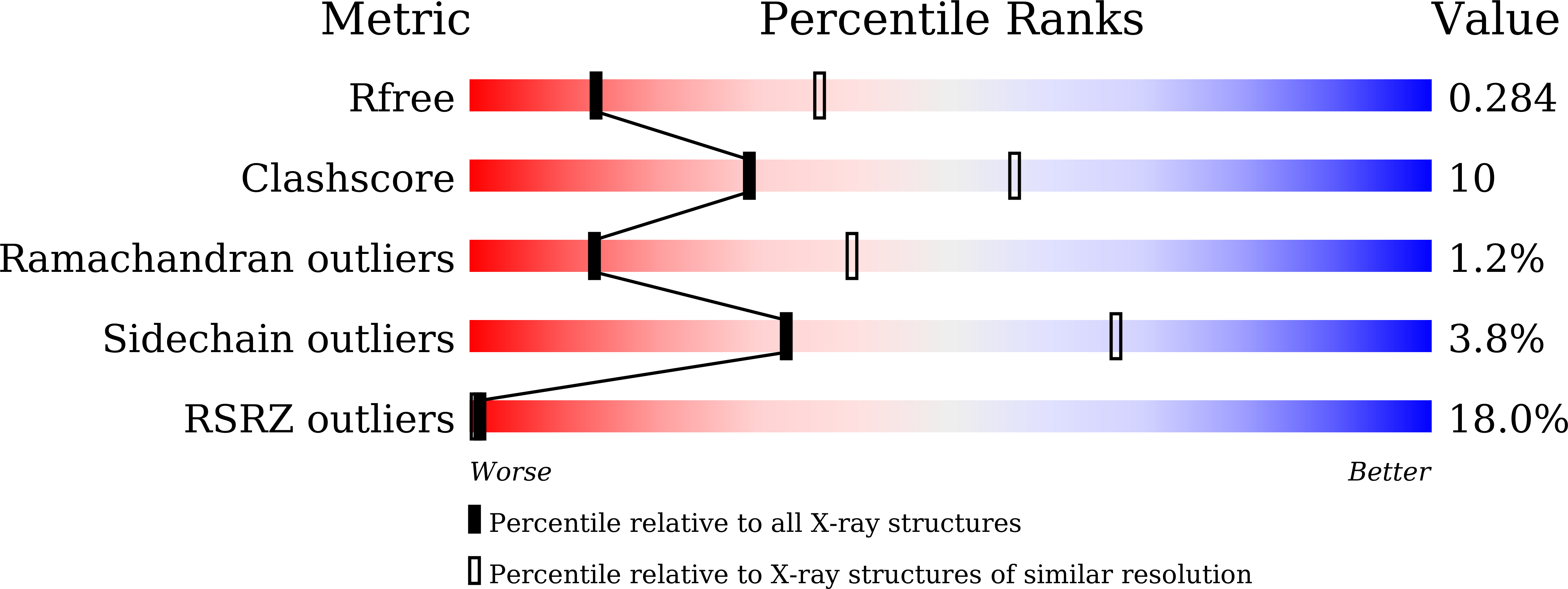
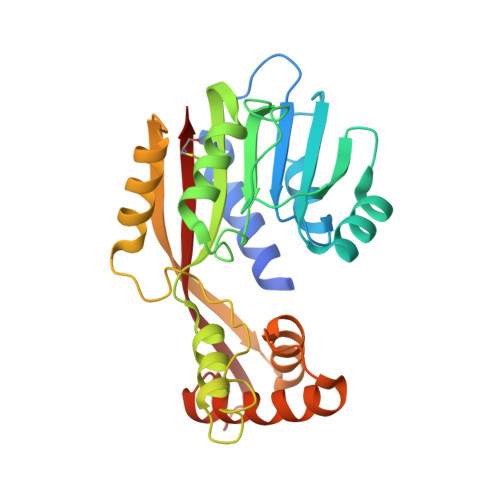
Structural basis for juvenile hormone biosynthesis by the juvenile hormone acid methyltransferase.
Guo, P., Zhang, Y., Zhang, L., Xu, H., Zhang, H., Wang, Z., Jiang, Y., Molloy, D., Zhao, P., Xia, Q.(2021) J Biol Chem 297: 101234-101234
- PubMed: 34562453
- DOI: https://doi.org/10.1016/j.jbc.2021.101234
- Primary Citation of Related Structures:
7EBS, 7EBX, 7EC0 - PubMed Abstract:
Juvenile hormone (JH) acid methyltransferase (JHAMT) is a rate-limiting enzyme that converts JH acids or inactive precursors of JHs to active JHs at the final step of JH biosynthesis in insects and thus presents an excellent target for the development of insect growth regulators or insecticides. However, the three-dimensional properties and catalytic mechanism of this enzyme are not known. Herein, we report the crystal structure of the JHAMT apoenzyme, the three-dimensional holoprotein in binary complex with its cofactor S-adenosyl-l-homocysteine, and the ternary complex with S-adenosyl-l-homocysteine and its substrate methyl farnesoate. These structures reveal the ultrafine definition of the binding patterns for JHAMT with its substrate/cofactor. Comparative structural analyses led to novel findings concerning the structural specificity of the progressive conformational changes required for binding interactions that are induced in the presence of cofactor and substrate. Importantly, structural and biochemical analyses enabled identification of one strictly conserved catalytic Gln/His pair within JHAMTs required for catalysis and further provide a molecular basis for substrate recognition and the catalytic mechanism of JHAMTs. These findings lay the foundation for the mechanistic understanding of JH biosynthesis by JHAMTs and provide a rational framework for the discovery and development of specific JHAMT inhibitors as insect growth regulators or insecticides.
Organizational Affiliation:
State Key Laboratory of Silkworm Genome Biology, Biological Science Research Center, Southwest University, Chongqing, China; Chongqing Key Laboratory of Sericultural Science, Chongqing Engineering and Technology Research Center for Novel Silk Materials, Southwest University, Chongqing, China.