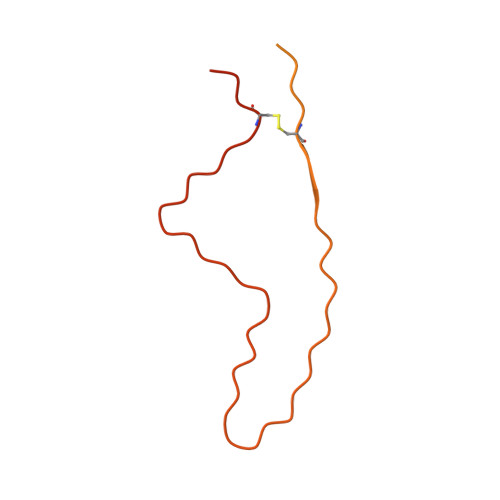
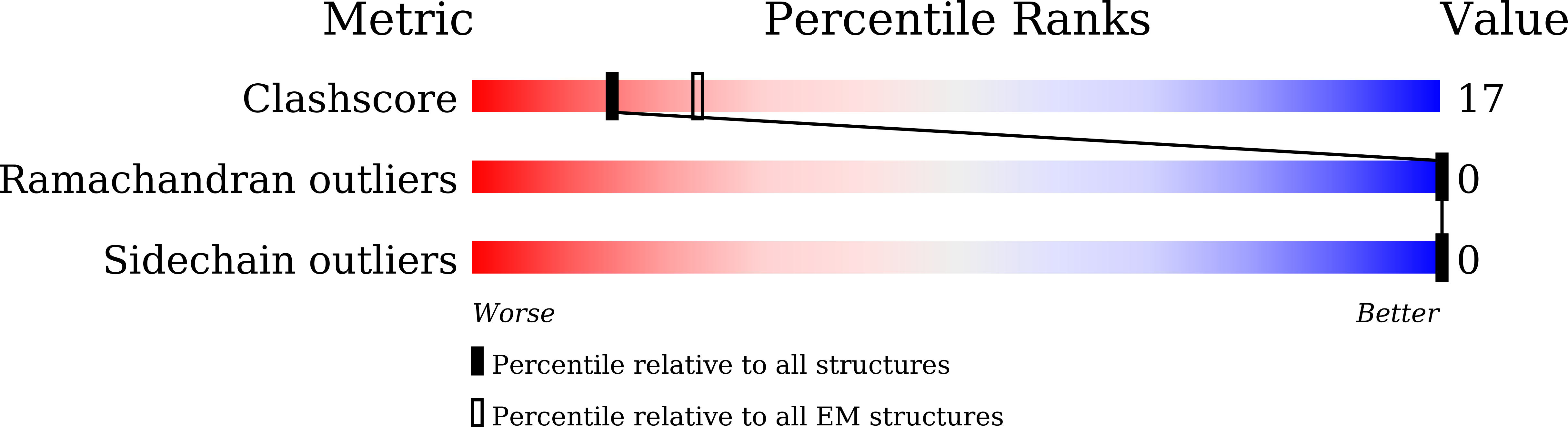Genetic prion disease-related mutation E196K displays a novel amyloid fibril structure revealed by cryo-EM.
Wang, L.Q., Zhao, K., Yuan, H.Y., Li, X.N., Dang, H.B., Ma, Y., Wang, Q., Wang, C., Sun, Y., Chen, J., Li, D., Zhang, D., Yin, P., Liu, C., Liang, Y.(2021) Sci Adv 7: eabg9676-eabg9676
- PubMed: 34516876
- DOI: https://doi.org/10.1126/sciadv.abg9676
- Primary Citation of Related Structures:
7DWV - PubMed Abstract:
Prion diseases are caused by the conformational conversion of prion protein (PrP). Forty-two different mutations were identified in human PrP, leading to genetic prion diseases with distinct clinical syndromes. Here, we report the cryo–electron microscopy structure of an amyloid fibril formed by full-length human PrP with E196K mutation, a genetic Creutzfeldt-Jakob disease–related mutation. This mutation disrupts key interactions in the wild-type PrP fibril, forming an amyloid fibril with a conformation distinct from the wild-type PrP fibril and hamster brain–derived prion fibril. The E196K fibril consists of two protofibrils. Each subunit forms five β strands stabilized by a disulfide bond and an unusual hydrophilic cavity stabilized by a salt bridge. Four pairs of amino acids from opposing subunits form four salt bridges to stabilize the zigzag interface of the two protofibrils. Our results provide structural evidences of the diverse prion strains and highlight the importance of familial mutations in inducing different strains.
Organizational Affiliation:
Hubei Key Laboratory of Cell Homeostasis, College of Life Sciences, Wuhan University, Wuhan 430072, China.