Phage-Display Based Discovery and Characterization of Peptide Ligands against WDR5.
Cao, J., Fan, T., Li, Y., Du, Z., Chen, L., Wang, Y., Wang, X., Shen, J., Huang, X., Xiong, B., Cao, D.(2021) Molecules 26
- PubMed: 33668971
- DOI: https://doi.org/10.3390/molecules26051225
- Primary Citation of Related Structures:
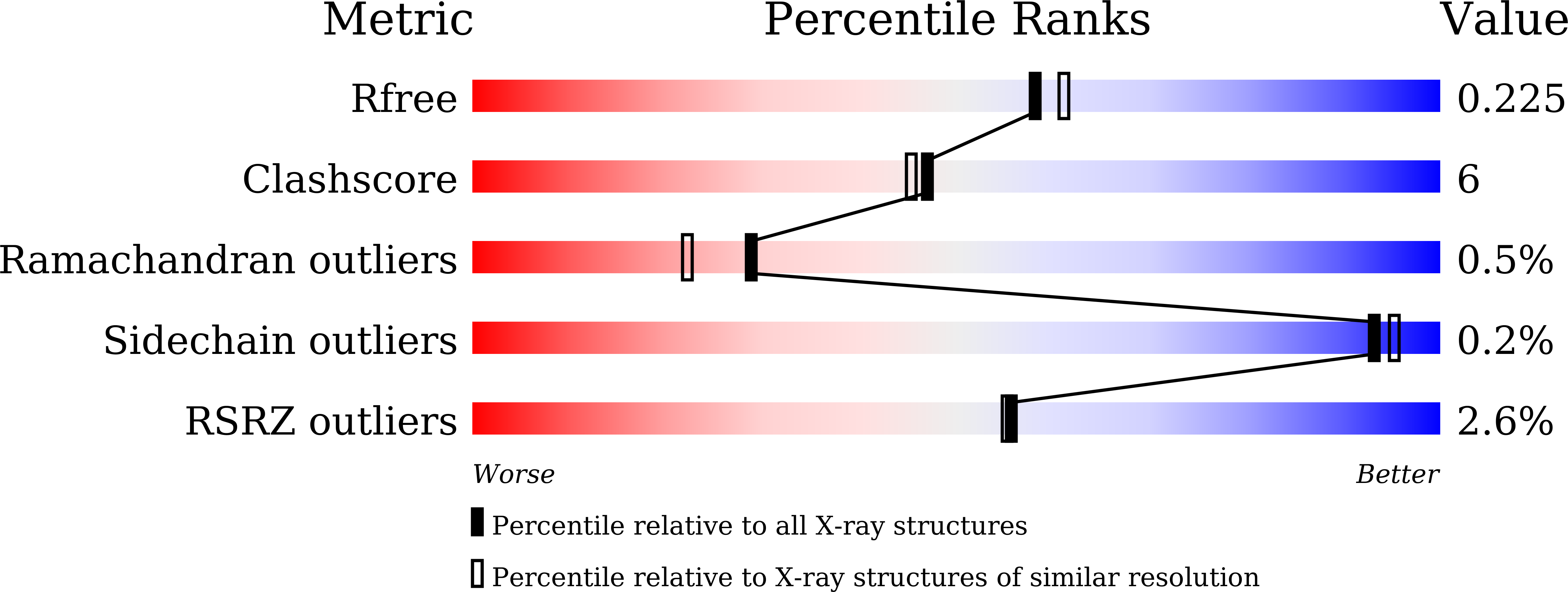
7DNO - PubMed Abstract:
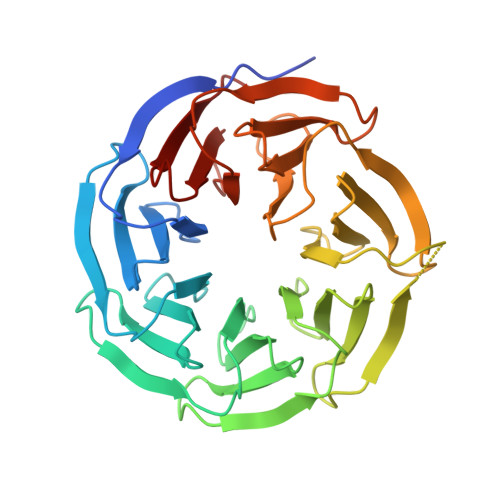
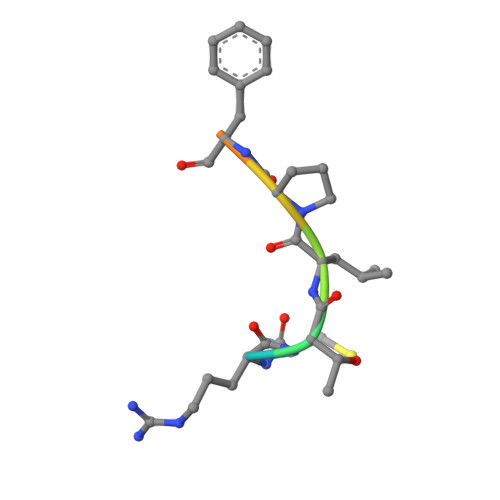
WD40 is a ubiquitous domain presented in at least 361 human proteins and acts as scaffold to form protein complexes. Among them, WDR5 protein is an important mediator in several protein complexes to exert its functions in histone modification and chromatin remodeling. Therefore, it was considered as a promising epigenetic target involving in anti-cancer drug development. In view of the protein-protein interaction nature of WDR5, we initialized a campaign to discover new peptide-mimic inhibitors of WDR5. In current study, we utilized the phage display technique and screened with a disulfide-based cyclic peptide phage library. Five rounds of biopanning were performed and isolated clones were sequenced. By analyzing the sequences, total five peptides were synthesized for binding assay. The four peptides are shown to have the moderate binding affinity. Finally, the detailed binding interactions were revealed by solving a WDR5-peptide cocrystal structure.
Organizational Affiliation:
Department of College of Pharmacy, University of Chinese Academy of Sciences, No. 19A Yuquan Road, Beijing 100049, China.