Structural basis for the metabolism of xenobiotic C-glycosides by intestinal bacteria
Mori, T., Kumano, T., He, K., Awakawa, T., Watanabe, S., Hori, S., Terashita, Y., Hashimoto, Y., Senda, M., Senda, T., Kobayashi, M., Abe, I.(2021) Nat Commun
Experimental Data Snapshot
(2021) Nat Commun
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| AP_endonuc_2 domain-containing protein | A, C [auth P] | 354 | Arthrobacter globiformis NBRC 12137 | Mutation(s): 0 Gene Names: ARGLB_075_00530 | 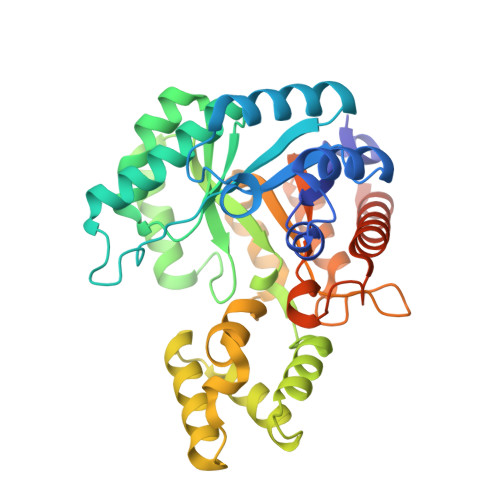 |
UniProt | |||||
Find proteins for H0QPL9 (Arthrobacter globiformis NBRC 12137) Explore H0QPL9 Go to UniProtKB: H0QPL9 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | H0QPL9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| AgCarC2 | B, D [auth Q] | 145 | Arthrobacter globiformis NBRC 12137 | Mutation(s): 0 Gene Names: ARGLB_075_00520 | 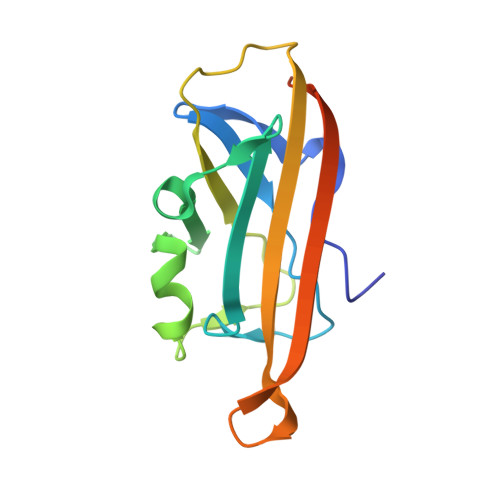 |
UniProt | |||||
Find proteins for H0QPL8 (Arthrobacter globiformis NBRC 12137) Explore H0QPL8 Go to UniProtKB: H0QPL8 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | H0QPL8 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| H9R (Subject of Investigation/LOI) Query on H9R | E [auth A] | 2-[3,4-bis(oxidanyl)phenyl]-6-[(2S,3R,4R,5S,6R)-6-(hydroxymethyl)-3,4,5-tris(oxidanyl)oxan-2-yl]-5,7-bis(oxidanyl)chromen-4-one C21 H20 O11 ODBRNZZJSYPIDI-VJXVFPJBSA-N |  | ||
| IOD Query on IOD | G [auth A], I [auth P] | IODIDE ION I XMBWDFGMSWQBCA-UHFFFAOYSA-M |  | ||
| MN (Subject of Investigation/LOI) Query on MN | F [auth A], H [auth P] | MANGANESE (II) ION Mn WAEMQWOKJMHJLA-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 73.905 | α = 90 |
| b = 102.511 | β = 90 |
| c = 136.09 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| XSCALE | data scaling |
| CRANK2 | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Japan Agency for Medical Research and Development (AMED) | Japan | JP19am0101001 |