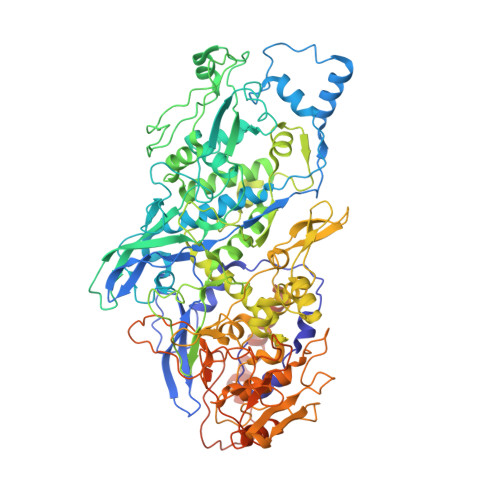
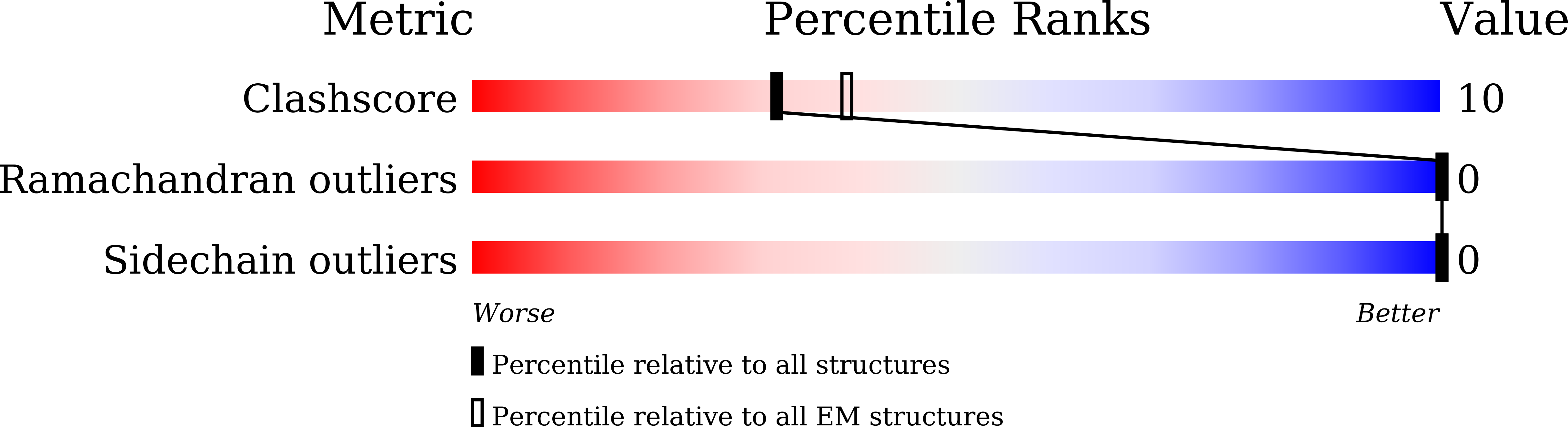Cryo-EM reveals a previously unrecognized structural protein of a dsRNA virus implicated in its extracellular transmission.
Shao, Q., Jia, X., Gao, Y., Liu, Z., Zhang, H., Tan, Q., Zhang, X., Zhou, H., Li, Y., Zhang, Q.(2021) PLoS Pathog 17: e1009396-e1009396
- PubMed: 33730056
- DOI: https://doi.org/10.1371/journal.ppat.1009396
- Primary Citation of Related Structures:
7CZ6, 7D0K, 7D0L - PubMed Abstract:
Mosquito viruses cause unpredictable outbreaks of disease. Recently, several unassigned viruses isolated from mosquitoes, including the Omono River virus (OmRV), were identified as totivirus-like viruses, with features similar to those of the Totiviridae family. Most reported members of this family infect fungi or protozoans and lack an extracellular life cycle stage. Here, we identified a new strain of OmRV and determined high-resolution structures for this virus using single-particle cryo-electron microscopy. The structures feature an unexpected protrusion at the five-fold vertex of the capsid. Disassociation of the protrusion could result in several conformational changes in the major capsid. All these structures, together with some biological results, suggest the protrusions' associations with the extracellular transmission of OmRV.
Organizational Affiliation:
State Key Lab for Biocontrol, School of Life Sciences, Sun Yat-sen University, Guangzhou, China.