Crystal structure of Arabinose isomerase from hyper thermophilic bacterium Thermotoga maritima (TMAI) wt
Hoang, N.K.Q., Dhanasingh, I., Cao, T.P., Sung, J.Y., Shin, S.M., Lee, D.W., Lee, S.H.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| L-arabinose isomerase | 497 | Geobacillus kaustophilus HTA426, Alicyclobacillus sp. TP7, Alicyclobacillus acidocaldarius subsp. acidocaldarius | Mutation(s): 0 Gene Names: araA, GK1904 EC: 5.3.1.4 | 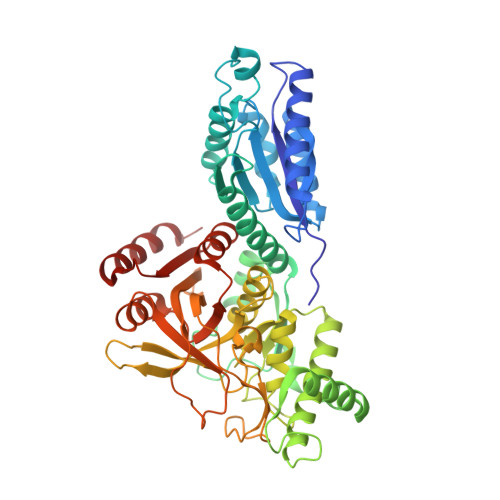 | |
UniProt | |||||
Find proteins for K0IGW6 (Alicyclobacillus sp. TP7) Explore K0IGW6 Go to UniProtKB: K0IGW6 | |||||
Find proteins for Q5KYP7 (Geobacillus kaustophilus (strain HTA426)) Explore Q5KYP7 Go to UniProtKB: Q5KYP7 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Groups | Q5KYP7K0IGW6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| MPD Query on MPD | G [auth A] H [auth A] K [auth B] M [auth C] N [auth C] | (4S)-2-METHYL-2,4-PENTANEDIOL C6 H14 O2 SVTBMSDMJJWYQN-YFKPBYRVSA-N |  | ||
| GOL Query on GOL | O [auth C] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| MN (Subject of Investigation/LOI) Query on MN | I [auth A] J [auth A] L [auth B] P [auth C] Q [auth C] | MANGANESE (II) ION Mn WAEMQWOKJMHJLA-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 204.591 | α = 90 |
| b = 81.907 | β = 117.9 |
| c = 192.001 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| MOLREP | phasing |