Structural insights into Pot1-ssDNA, Pot1-Tpz1 and Tpz1-Ccq1 Interactions within fission yeast shelterin complex.
Sun, H., Wu, Z., Zhou, Y., Lu, Y., Lu, H., Chen, H., Shi, S., Zeng, Z., Wu, J., Lei, M.(2022) PLoS Genet 18: e1010308-e1010308
- PubMed: 35849625
- DOI: https://doi.org/10.1371/journal.pgen.1010308
- Primary Citation of Related Structures:
7CUH, 7CUI, 7CUJ - PubMed Abstract:
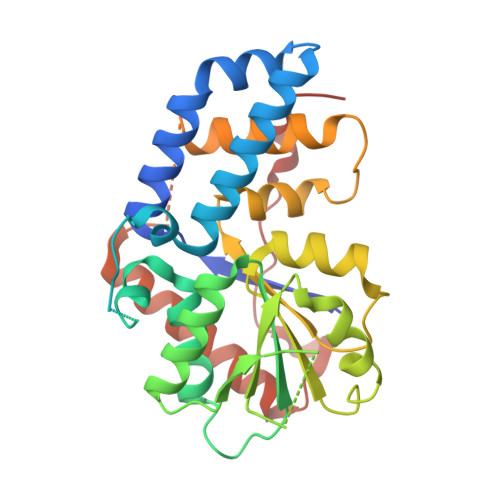
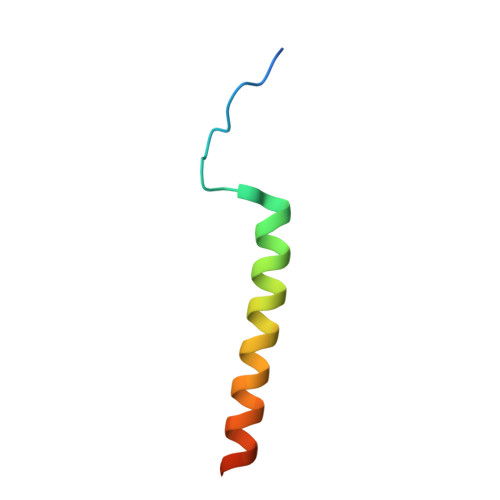
The conserved shelterin complex caps chromosome ends to protect telomeres and regulate telomere replication. In fission yeast Schizosaccharomyces pombe, shelterin consists of telomeric single- and double-stranded DNA-binding modules Pot1-Tpz1 and Taz1-Rap1 connected by Poz1, and a specific component Ccq1. While individual structures of the two DNA-binding OB folds of Pot1 (Pot1OB1-GGTTAC and Pot1OB2-GGTTACGGT) are available, structural insight into recognition of telomeric repeats with spacers by the complete DNA-binding domain (Pot1DBD) remains an open question. Moreover, structural information about the Tpz1-Ccq1 interaction requires to be revealed for understanding how the specific component Ccq1 of S. pombe shelterin is recruited to telomeres to function as an interacting hub. Here, we report the crystal structures of Pot1DBD-single-stranded-DNA, Pot1372-555-Tpz1185-212 and Tpz1425-470-Ccq1123-439 complexes and propose an integrated model depicting the assembly mechanism of the shelterin complex at telomeres. The structure of Pot1DBD-DNA unveils how Pot1 recognizes S. pombe degenerate telomeric sequences. Our analyses of Tpz1-Ccq1 reveal structural basis for the essential role of the Tpz1-Ccq1 interaction in telomere recruitment of Ccq1 that is required for telomere maintenance and telomeric heterochromatin formation. Overall, our findings provide valuable structural information regarding interactions within fission yeast shelterin complex at 3' ss telomeric overhang.
Organizational Affiliation:
State Key Laboratory of Molecular Biology, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai, China.