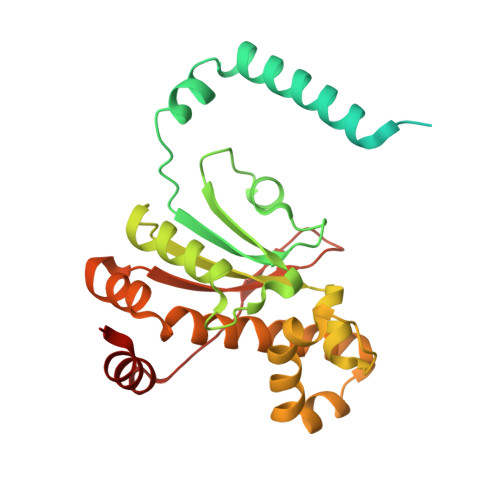
Crystal Structure of beta-Carbonic Anhydrase CafA from the Fungal Pathogen Aspergillus fumigatus .
Kim, S., Yeon, J., Sung, J., Jin, M.S.(2020) Mol Cells 43: 831-840
- PubMed: 32975213
- DOI: https://doi.org/10.14348/molcells.2020.0168
- Primary Citation of Related Structures:
7COI, 7COJ - PubMed Abstract:
The β-class of carbonic anhydrases (β-CAs) are zinc metalloenzymes widely distributed in the fungal kingdom that play essential roles in growth, survival, differentiation, and virulence by catalyzing the reversible interconversion of carbon dioxide (CO 2 ) and bicarbonate (HCO 3 - ). Herein, we report the biochemical and crystallographic characterization of the β-CA CafA from the fungal pathogen Aspergillus fumigatus , the main causative agent of invasive aspergillosis. CafA exhibited apparent in vitro CO 2 hydration activity in neutral to weak alkaline conditions, but little activity at acidic pH. The high-resolution crystal structure of CafA revealed a tetramer comprising a dimer of dimers, in which the catalytic zinc ion is tetrahedrally coordinated by three conserved residues (C119, H175, C178) and an acetate anion presumably acquired from the crystallization solution, indicating a freely accessible ″open″ conformation. Furthermore, knowledge of the structure of CafA in complex with the potent inhibitor acetazolamide, together with its functional intolerance of nitrate (NO 3 - ) ions, could be exploited to develop new antifungal agents for the treatment of invasive aspergillosis.
Organizational Affiliation:
School of Life Sciences, Gwangju Institute of Science and Technology (GIST), Gwangju 61005, Korea.