Structure-based analysis and evolution of a monomerized red-colored chromoprotein from the Olindias formosa jellyfish.
Zhai, L., Nakashima, R., Shinoda, H., Ike, Y., Matsuda, T., Nagai, T.(2022) Protein Sci 31: e4285-e4285
- PubMed: 35481635
- DOI: https://doi.org/10.1002/pro.4285
- Primary Citation of Related Structures:
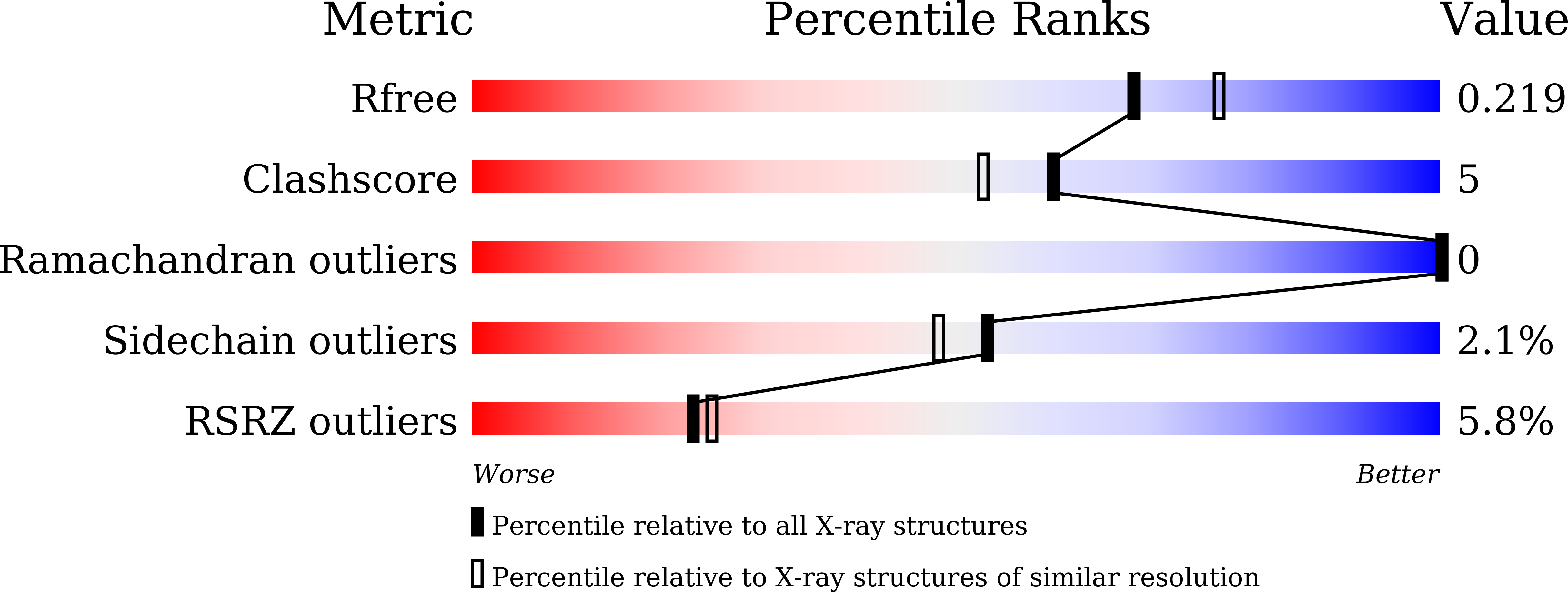
7CAO - PubMed Abstract:
GFP-like chromoproteins (CPs) with non-fluorescence ability have been used as bioimaging probes. Existing CPs have voids in the optical absorption window which limits their extensibility. The development of new CP color is therefore ongoing. Here, we cloned CPs from the jellyfish, Olindias formosa, and developed a completely non-fluorescent monomeric red CP, R-Velour, with an absorption peak at 528 nm. To analyze the photophysical properties from a structural aspect, we determined the crystal structure of R-Velour at a 2.1 Å resolution. R-Velour has a trans-chromophore similar to the green fluorescence protein, Gamillus, derived from the same jellyfish. However, in contrast to the two coplanar chromophoric rings in Gamillus, R-Velour has a large torsion inducing non-fluorescence property. Through site-directed mutagenesis, we surveyed residues surrounding the chromophore and found a key residue, Ser155, which contributes to the generation of four-color variants with the bathochromic and hypsochromic shift of the absorption peak, ranging from 506 to 554 nm. The recently proposed spectrum shift theory, based on the Marcus-Hush model, supports the spectrum shift of these mutants. These findings may support further development of R-Velour variants with useful absorption characteristics for bioimaging, including fluorescence lifetime imaging and photoacoustic imaging.
Organizational Affiliation:
Graduate School of Frontier Bioscience, Osaka University, Suita, Japan.