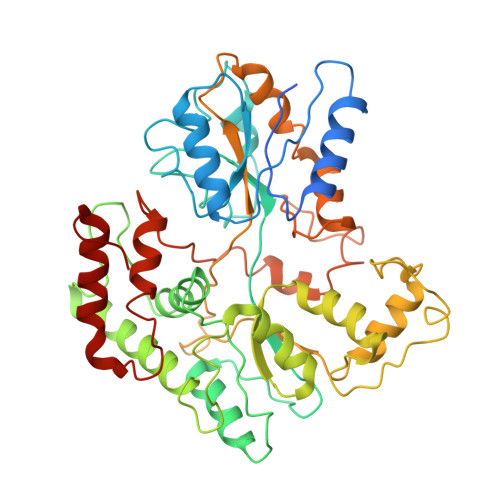
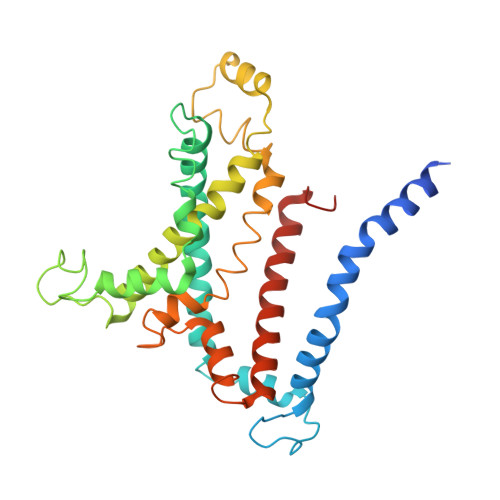
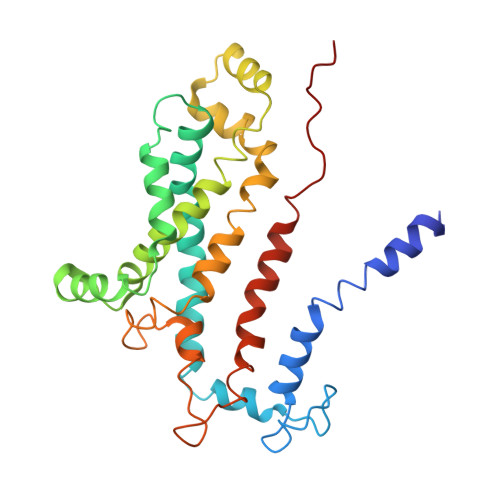
Structural basis of trehalose recycling by the ABC transporter LpqY-SugABC.
Liu, F., Liang, J., Zhang, B., Gao, Y., Yang, X., Hu, T., Yang, H., Xu, W., Guddat, L.W., Rao, Z.(2020) Sci Adv 6
- PubMed: 33127676
- DOI: https://doi.org/10.1126/sciadv.abb9833
- Primary Citation of Related Structures:
7CAD, 7CAE, 7CAF, 7CAG - PubMed Abstract:
In bacteria, adenosine 5'-triphosphate (ATP)-binding cassette (ABC) importers are essential for the uptake of nutrients including the nonreducing disaccharide trehalose, a metabolite that is crucial for the survival and virulence of several human pathogens including Mycobacterium tuberculosis SugABC is an ABC transporter that translocates trehalose from the periplasmic lipoprotein LpqY into the cytoplasm of mycobacteria. Here, we report four high-resolution cryo-electron microscopy structures of the mycobacterial LpqY-SugABC complex to reveal how it binds and passes trehalose through the membrane to the cytoplasm. A unique feature observed in this system is the initial mode of capture of the trehalose at the LpqY interface. Uptake is achieved by a pivotal rotation of LpqY relative to SugABC, moving from an open and accessible conformation to a clamped conformation upon trehalose binding. These findings enrich our understanding as to how ABC transporters facilitate substrate transport across the membrane in Gram-positive bacteria.
Organizational Affiliation:
Shanghai Institute for Advanced Immunochemical Studies and School of Life Science and Technology, ShanghaiTech University, Shanghai 201210, China.