Crystal structure of the N-terminal domain of human MdmX protein in complex with Nutlin3a
Su, Z.D., Cheng, X.Y., Zhang, B.L., Kuang, Z.K., Yang, J., Li, Z.C., Yu, J.P., Cao, C.Z., Zhao, Z.T.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Protein Mdm4 | 96 | Homo sapiens | Mutation(s): 0 Gene Names: MDM4, MDMX | 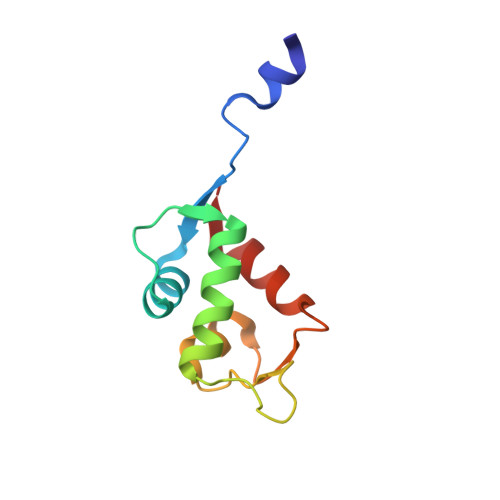 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O15151 (Homo sapiens) Explore O15151 Go to UniProtKB: O15151 | |||||
PHAROS: O15151 GTEx: ENSG00000198625 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O15151 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NUT (Subject of Investigation/LOI) Query on NUT | C [auth A] | 4-({(4S,5R)-4,5-bis(4-chlorophenyl)-2-[4-methoxy-2-(propan-2-yloxy)phenyl]-4,5-dihydro-1H-imidazol-1-yl}carbonyl)piperazin-2-one C30 H30 Cl2 N4 O4 BDUHCSBCVGXTJM-WUFINQPMSA-N |  | ||
| O4B Query on O4B | B [auth A] | 1,4,7,10,13,16-HEXAOXACYCLOOCTADECANE C12 H24 O6 XEZNGIUYQVAUSS-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 47.552 | α = 90 |
| b = 47.552 | β = 90 |
| c = 90.676 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |