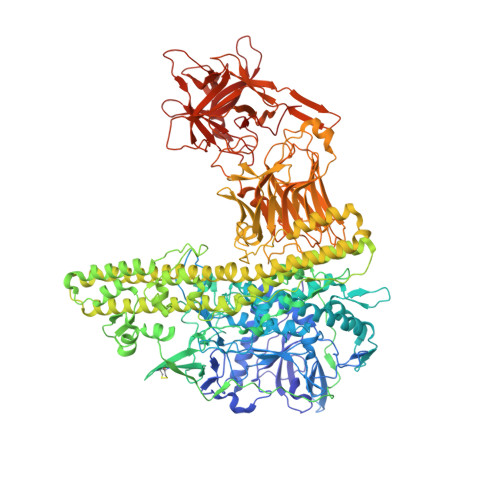
Structural flexibility of the tetanus neurotoxin revealed by crystallographic and solution scattering analyses.
Zhang, C.M., Imoto, Y., Hikima, T., Inoue, T.(2021) J Struct Biol X 5: 100045-100045
- PubMed: 33598655
- DOI: https://doi.org/10.1016/j.yjsbx.2021.100045
- Primary Citation of Related Structures:
7BXX, 7BY4, 7BY5 - PubMed Abstract:
Although the tetanus neurotoxin (TeNT) delivers its protease domain (LC) across the synaptic vesicle lumen into the cytosol via its receptor binding domain (H C ) and translocation domain (H N ), the molecular mechanism coordinating this membrane translocation remains unresolved. Here, we report the high-resolution crystal structures of full-length reduced TeNT (rTeNT, 2.3 Å), TeNT isolated H N (TeNT/iH N , 2.3 Å), TeNT isolated H C (TeNT/iH C , 1.5 Å), together with the solution structures of TeNT/iH N and beltless TeNT/iH N (TeNT/blH N ). TeNT undergoes significant domains rotation of the H N and LC were demonstrated by structural comparison of rTeNT and non-reduced-TeNT (nrTeNT). A linker loop connects the H N and H C is essential for the self-domain rotation of TeNT. The TeNT-specific C869-C1093 disulfide bond is sensitive to the redox environment and its disruption provides linker loop flexibility, which enables domain arrangement of rTeNT distinct from that of nrTeNT. Furthermore, the mobility of C869 in the linker loop and the sensitivity to redox condition of C1093 were confirmed by crystal structure analysis of TeNT/iH C . On the other hand, the structural flexibility of H N was investigated by crystallographic and solution scattering analyses. It was found that the region (residues 698-769), which follows the translocation region had remarkable change in TeNT/iH N . Besides, the so-called belt region has a high propensity to swing around the upper half of TeNT/iH N at acidic pH. It provides the first overview of the dynamics of the Belt in solution. These newly obtained structural information that shed light on the transmembrane mechanism of TeNT.
Organizational Affiliation:
Graduate School of Pharmaceutical Science, Osaka University, Suita, 565-0871 Osaka, Japan.