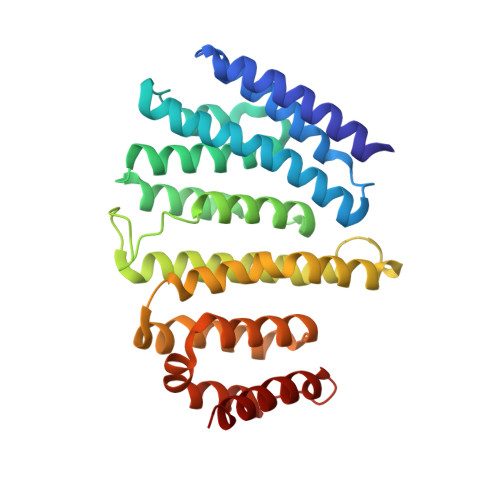
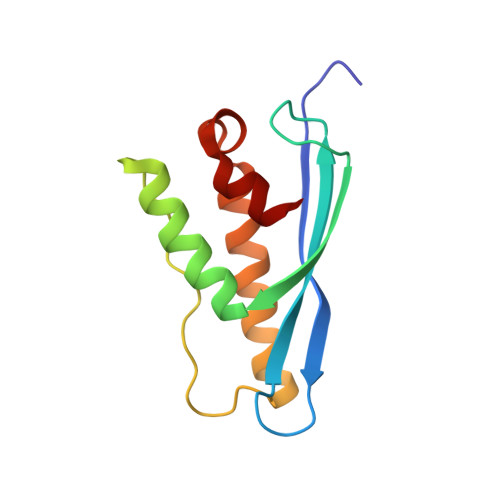
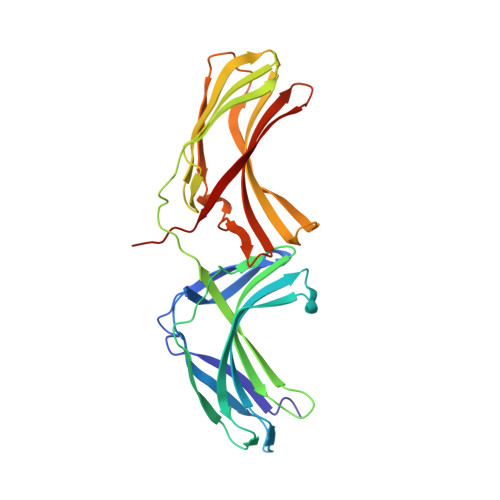
Architecture and mechanism of metazoan retromer:SNX3 tubular coat assembly.
Leneva, N., Kovtun, O., Morado, D.R., Briggs, J.A.G., Owen, D.J.(2021) Sci Adv 7
- PubMed: 33762348
- DOI: https://doi.org/10.1126/sciadv.abf8598
- Primary Citation of Related Structures:
7BLN, 7BLO, 7BLP, 7BLQ, 7BLR - PubMed Abstract:
Retromer is a master regulator of cargo retrieval from endosomes, which is critical for many cellular processes including signaling, immunity, neuroprotection, and virus infection. The retromer core (VPS26/VPS29/VPS35) is present on cargo-transporting, tubular carriers along with a range of sorting nexins. Here, we elucidate the structural basis of membrane tubulation and coupled cargo recognition by metazoan and fungal retromer coats assembled with the non-Bin1/Amphiphysin/Rvs (BAR) sorting nexin SNX3 using cryo-electron tomography. The retromer core retains its arched, scaffolding structure but changes its mode of membrane recruitment when assembled with different SNX adaptors, allowing cargo recognition at subunit interfaces. Thus, membrane bending and cargo incorporation can be modulated to allow retromer to traffic cargoes along different cellular transport routes.
Organizational Affiliation:
Cambridge Institute for Medical Research, Cambridge Biomedical Campus, Hills Road, Cambridge CB2 0XY, UK. nl365@cam.ac.uk okovtun@mrc-lmb.cam.ac.uk jbriggs@mrc-lmb.cam.ac.uk djo30@cam.ac.uk.