An on-demand, drop-on-drop method for studying enzyme catalysis by serial crystallography.
Butryn, A., Simon, P.S., Aller, P., Hinchliffe, P., Massad, R.N., Leen, G., Tooke, C.L., Bogacz, I., Kim, I.S., Bhowmick, A., Brewster, A.S., Devenish, N.E., Brem, J., Kamps, J.J.A.G., Lang, P.A., Rabe, P., Axford, D., Beale, J.H., Davy, B., Ebrahim, A., Orlans, J., Storm, S.L.S., Zhou, T., Owada, S., Tanaka, R., Tono, K., Evans, G., Owen, R.L., Houle, F.A., Sauter, N.K., Schofield, C.J., Spencer, J., Yachandra, V.K., Yano, J., Kern, J.F., Orville, A.M.(2021) Nat Commun 12: 4461-4461
- PubMed: 34294694
- DOI: https://doi.org/10.1038/s41467-021-24757-7
- Primary Citation of Related Structures:
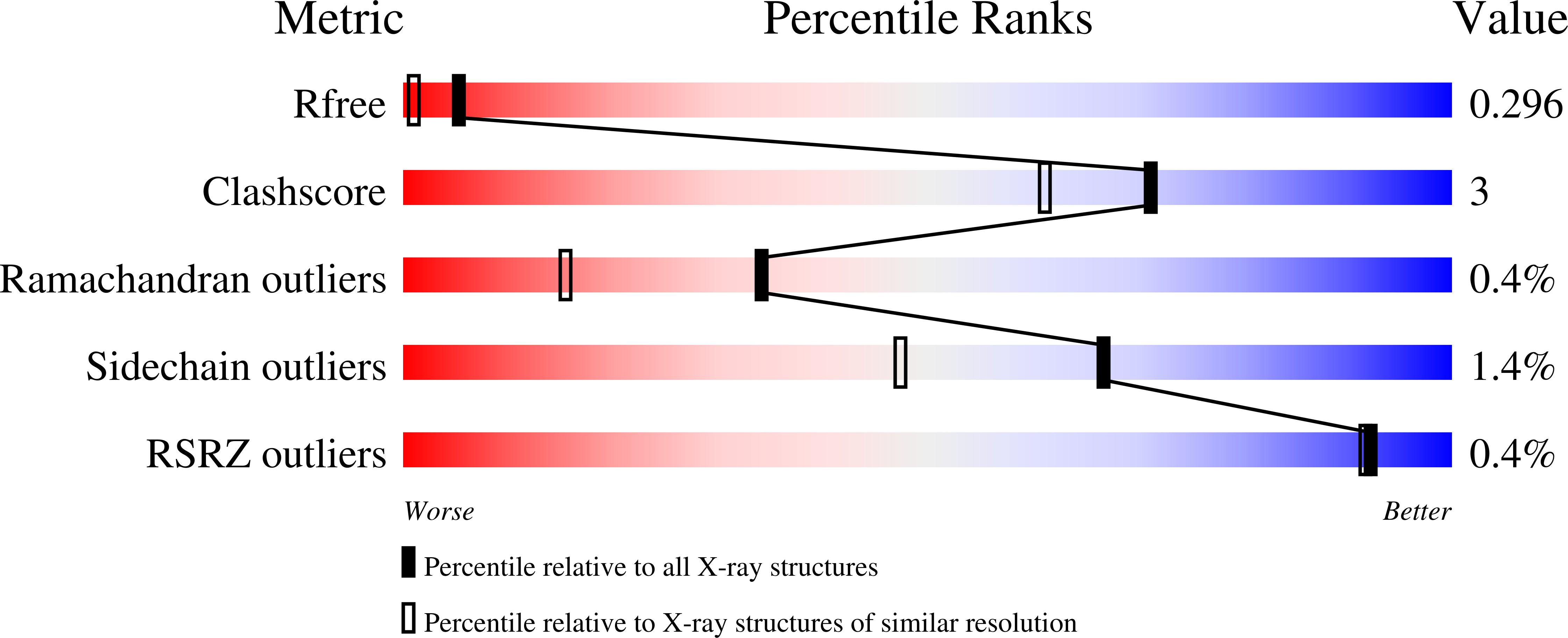
7BH3, 7BH4, 7BH5, 7BH6, 7BH7, 7BHK, 7BHL, 7BHM, 7BHN - PubMed Abstract:
Serial femtosecond crystallography has opened up many new opportunities in structural biology. In recent years, several approaches employing light-inducible systems have emerged to enable time-resolved experiments that reveal protein dynamics at high atomic and temporal resolutions. However, very few enzymes are light-dependent, whereas macromolecules requiring ligand diffusion into an active site are ubiquitous. In this work we present a drop-on-drop sample delivery system that enables the study of enzyme-catalyzed reactions in microcrystal slurries. The system delivers ligand solutions in bursts of multiple picoliter-sized drops on top of a larger crystal-containing drop inducing turbulent mixing and transports the mixture to the X-ray interaction region with temporal resolution. We demonstrate mixing using fluorescent dyes, numerical simulations and time-resolved serial femtosecond crystallography, which show rapid ligand diffusion through microdroplets. The drop-on-drop method has the potential to be widely applicable to serial crystallography studies, particularly of enzyme reactions with small molecule substrates.
Organizational Affiliation:
Diamond Light Source, Harwell Science and Innovation Campus, Didcot, UK.