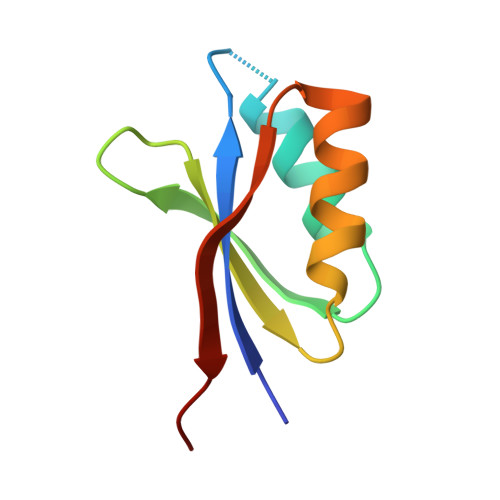
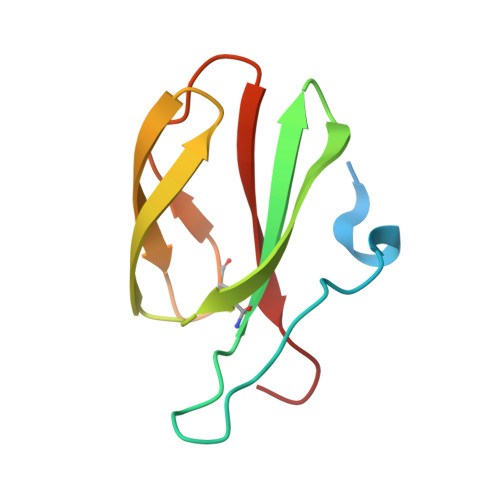
Multiple variants of the fungal effector AVR-Pik bind the HMA domain of the rice protein OsHIPP19, providing a foundation to engineer plant defense.
Maidment, J.H.R., Franceschetti, M., Maqbool, A., Saitoh, H., Jantasuriyarat, C., Kamoun, S., Terauchi, R., Banfield, M.J.(2021) J Biol Chem 296: 100371-100371
- PubMed: 33548226
- DOI: https://doi.org/10.1016/j.jbc.2021.100371
- Primary Citation of Related Structures:
7B1I - PubMed Abstract:
Microbial plant pathogens secrete effector proteins, which manipulate the host to promote infection. Effectors can be recognized by plant intracellular nucleotide-binding leucine-rich repeat (NLR) receptors, initiating an immune response. The AVR-Pik effector from the rice blast fungus Magnaporthe oryzae is recognized by a pair of rice NLR receptors, Pik-1 and Pik-2. Pik-1 contains a noncanonical integrated heavy-metal-associated (HMA) domain, which directly binds AVR-Pik to activate plant defenses. The host targets of AVR-Pik are also HMA-domain-containing proteins, namely heavy-metal-associated isoprenylated plant proteins (HIPPs) and heavy-metal-associated plant proteins (HPPs). Here, we demonstrate that one of these targets interacts with a wider set of AVR-Pik variants compared with the Pik-1 HMA domains. We define the biochemical and structural basis of the interaction between AVR-Pik and OsHIPP19 and compare the interaction to that formed with the HMA domain of Pik-1. Using analytical gel filtration and surface plasmon resonance, we show that multiple AVR-Pik variants, including the stealthy variants AVR-PikC and AVR-PikF, which do not interact with any characterized Pik-1 alleles, bind to OsHIPP19 with nanomolar affinity. The crystal structure of OsHIPP19 in complex with AVR-PikF reveals differences at the interface that underpin high-affinity binding of OsHIPP19-HMA to a wider set of AVR-Pik variants than achieved by the integrated HMA domain of Pik-1. Our results provide a foundation for engineering the HMA domain of Pik-1 to extend binding to currently unrecognized AVR-Pik variants and expand disease resistance in rice to divergent pathogen strains.
Organizational Affiliation:
Department of Biological Chemistry, John Innes Centre, Norwich Research Park, Norwich, UK.