Structural determinants of DNA recognition by the NO sensor NsrR and related Rrf2-type [FeS]-transcription factors.
Rohac, R., Crack, J.C., de Rosny, E., Gigarel, O., Le Brun, N.E., Fontecilla-Camps, J.C., Volbeda, A.(2022) Commun Biol 5: 769-769
- PubMed: 35908109
- DOI: https://doi.org/10.1038/s42003-022-03745-7
- Primary Citation of Related Structures:
7B0C - PubMed Abstract:
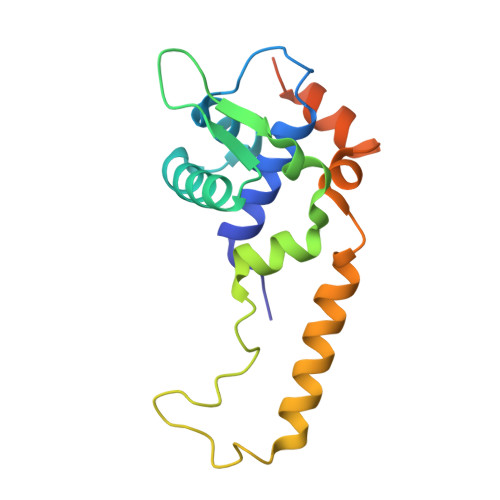
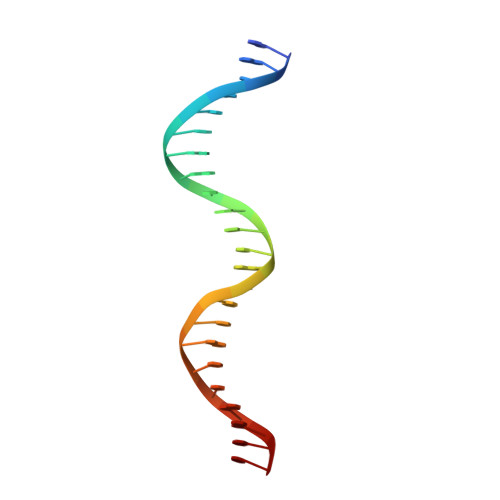
Several transcription factors of the Rrf2 family use an iron-sulfur cluster to regulate DNA binding through effectors such as nitric oxide (NO), cellular redox status and iron levels. [4Fe-4S]-NsrR from Streptomyces coelicolor (ScNsrR) modulates expression of three different genes via reaction and complex formation with variable amounts of NO, which results in detoxification of this gas. Here, we report the crystal structure of ScNsrR complexed with an hmpA1 gene operator fragment and compare it with those previously reported for [2Fe-2S]-RsrR/rsrR and apo-IscR/hyA complexes. Important structural differences reside in the variation of the DNA minor and major groove widths. In addition, different DNA curvatures and different interactions with the protein sensors are observed. We also report studies of NsrR binding to four hmpA1 variants, which indicate that flexibility in the central region is not a key binding determinant. Our study explores the promotor binding specificities of three closely related transcriptional regulators.
Organizational Affiliation:
Univ. Grenoble Alpes, CEA, CNRS, Institut de Biologie Structurale, Metalloproteins Unit, F-38000, Grenoble, France.