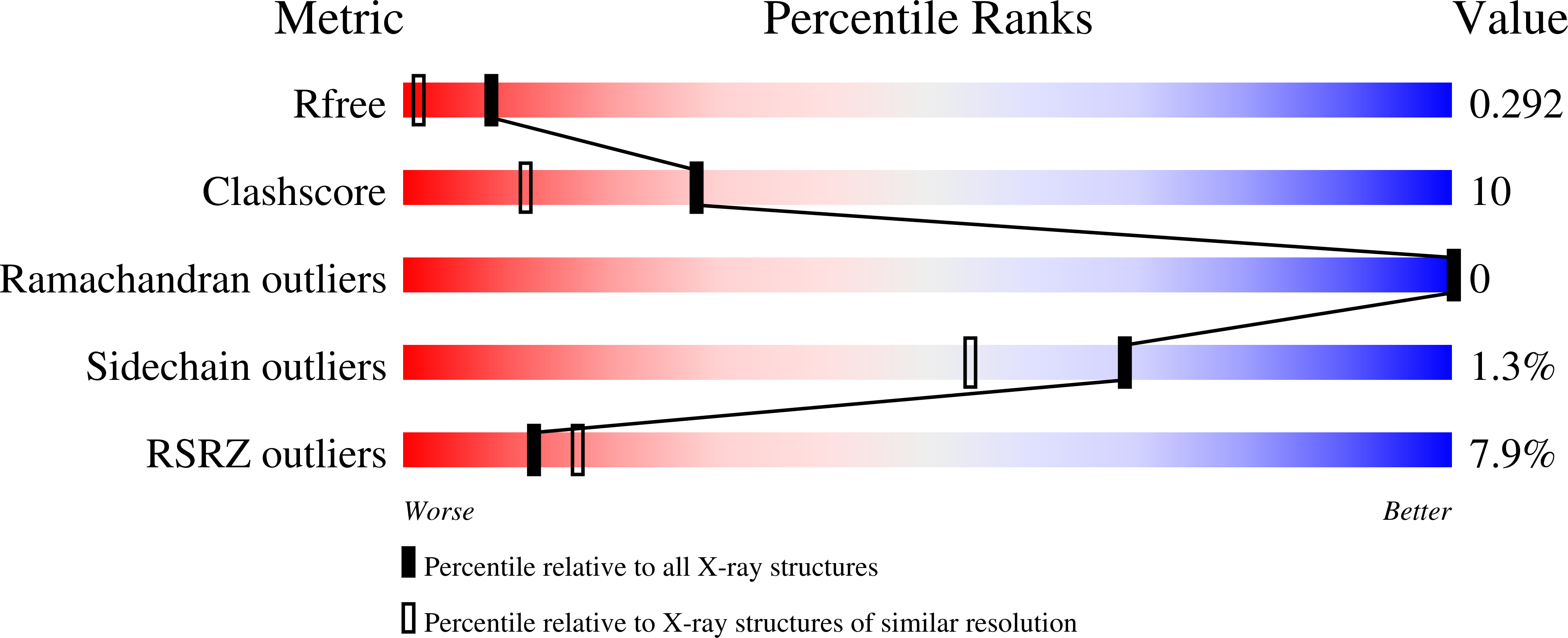
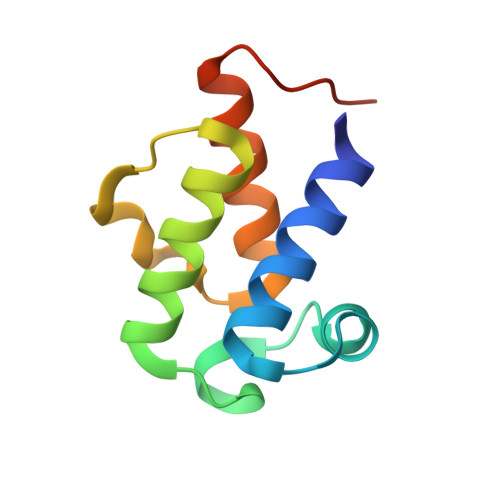
Dynamics in an unusual acyl carrier protein from a ladderane lipid-synthesizing organism.
Dietl, A., Barends, T.R.M.(2022) Proteins 90: 73-82
- PubMed: 34310758
- DOI: https://doi.org/10.1002/prot.26187
- Primary Citation of Related Structures:
7AUF, 7AX5 - PubMed Abstract:
Anaerobic ammonium-oxidizing (anammox) bacteria express a distinct acyl carrier protein implicated in the biosynthesis of the highly unusual "ladderane" lipids these organisms produce. This "anammox-specific" ACP, or amxACP, shows several unique features such as a conserved FF motif and an unusual sequence in the functionally important helix III. Investigation of the protein's structure and dynamics, both in the crystal by ensemble refinement and by MD simulations, reveals that helix III adopts a rare six-residue-long 3 10 -helical conformation that confers a large degree of conformational and positional variability on this part of the protein. This way of introducing structural flexibility by using the inherent properties of 3 10 -helices appears unique among ACPs. Moreover, the structure suggests a role for the FF motif in shielding the thioester linkage between the protein's prosthetic group and its acyl cargo from hydrolysis.
Organizational Affiliation:
Department of Biomolecular Mechanisms, Max Planck Institute for Medical Research, Heidelberg, Germany.