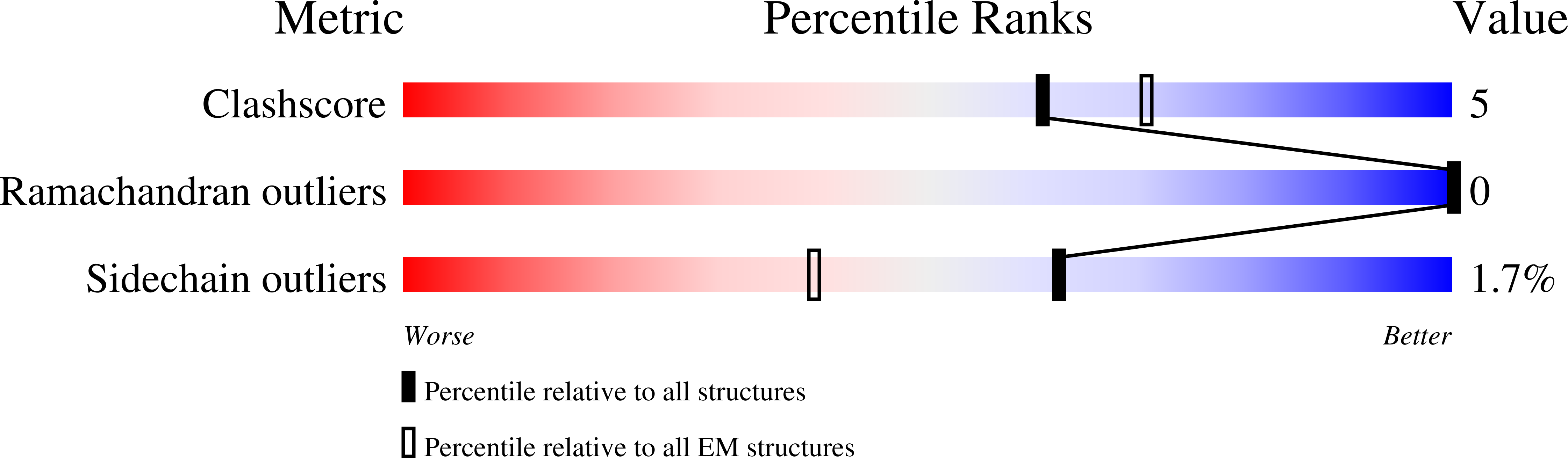Structure of native glycolipoprotein filaments in honeybee royal jelly.
Mattei, S., Ban, A., Picenoni, A., Leibundgut, M., Glockshuber, R., Boehringer, D.(2020) Nat Commun 11: 6267-6267
- PubMed: 33293513
- DOI: https://doi.org/10.1038/s41467-020-20135-x
- Primary Citation of Related Structures:
7ASD - PubMed Abstract:
Royal jelly (RJ) is produced by honeybees (Apis mellifera) as nutrition during larval development. The high viscosity of RJ originates from high concentrations of long lipoprotein filaments that include the glycosylated major royal jelly protein 1 (MRJP1), the small protein apisimin and insect lipids. Using cryo-electron microscopy we reveal the architecture and the composition of RJ filaments, in which the MRJP1 forms the outer shell of the assembly, surrounding stacked apisimin tetramers harbouring tightly packed lipids in the centre. The structural data rationalize the pH-dependent disassembly of RJ filaments in the gut of the larvae.
Organizational Affiliation:
Department of Biology, Institute of Molecular Biology and Biophysics, ETH Zurich, Otto-Stern-Weg 5, Zurich, 8093, Switzerland.