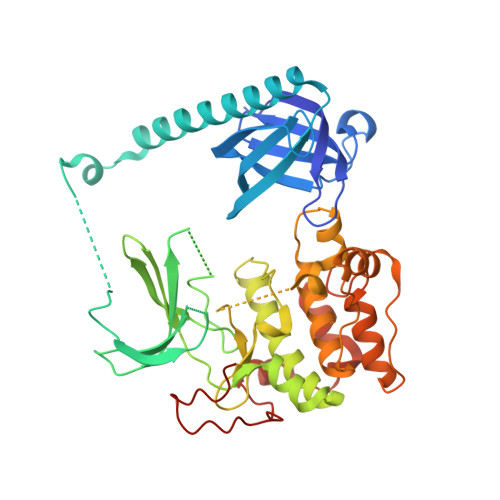
Structure of autoinhibited Akt1 reveals mechanism of PIP 3 -mediated activation.
Truebestein, L., Hornegger, H., Anrather, D., Hartl, M., Fleming, K.D., Stariha, J.T.B., Pardon, E., Steyaert, J., Burke, J.E., Leonard, T.A.(2021) Proc Natl Acad Sci U S A 118
- PubMed: 34385319
- DOI: https://doi.org/10.1073/pnas.2101496118
- Primary Citation of Related Structures:
7APJ - PubMed Abstract:
The protein kinase Akt is one of the primary effectors of growth factor signaling in the cell. Akt responds specifically to the lipid second messengers phosphatidylinositol-3,4,5-trisphosphate [PI(3,4,5)P 3 ] and phosphatidylinositol-3,4-bisphosphate [PI(3,4)P 2 ] via its PH domain, leading to phosphorylation of its activation loop and the hydrophobic motif of its kinase domain, which are critical for activity. We have now determined the crystal structure of Akt1, revealing an autoinhibitory interface between the PH and kinase domains that is often mutated in cancer and overgrowth disorders. This interface persists even after stoichiometric phosphorylation, thereby restricting maximum Akt activity to PI(3,4,5)P 3 - or PI(3,4)P 2 -containing membranes. Our work helps to resolve the roles of lipids and phosphorylation in the activation of Akt and has wide implications for the spatiotemporal control of Akt and potentially lipid-activated kinase signaling in general.
Organizational Affiliation:
Department of Structural and Computational Biology, Max Perutz Labs, Vienna BioCenter, 1030 Vienna, Austria.