Discovery of a Novel Class of Covalent Dual Inhibitors Targeting the Protein Kinases BMX and BTK.
Forster, M., Liang, X.J., Schroder, M., Gerstenecker, S., Chaikuad, A., Knapp, S., Laufer, S., Gehringer, M.(2020) Int J Mol Sci 21
- PubMed: 33291717
- DOI: https://doi.org/10.3390/ijms21239269
- Primary Citation of Related Structures:
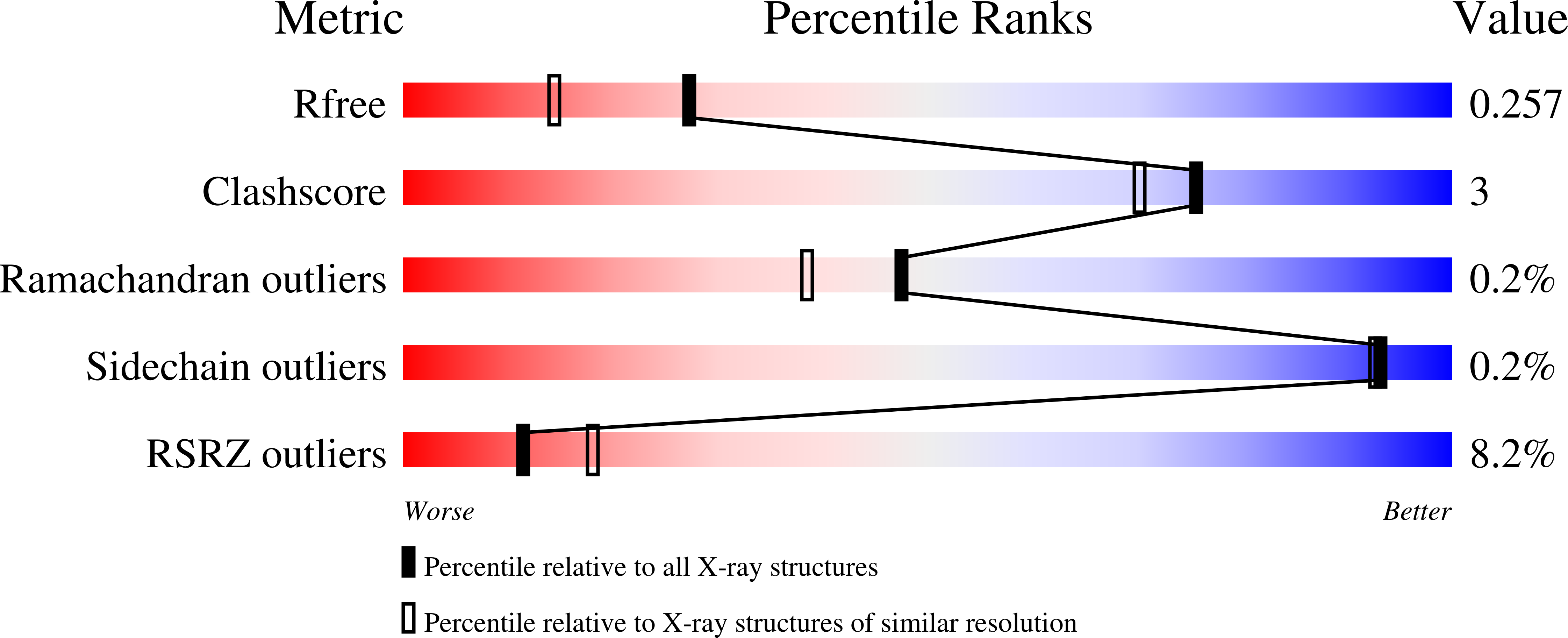
7APF, 7APG - PubMed Abstract:
The nonreceptor tyrosine TEC kinases are key regulators of the immune system and play a crucial role in the pathogenesis of diverse hematological malignancies. In contrast to the substantial efforts in inhibitor development for Bruton's tyrosine kinase (BTK), specific inhibitors of the other TEC kinases, including the bone marrow tyrosine kinase on chromosome X (BMX), remain sparse. Here we present a novel class of dual BMX/BTK inhibitors, which were designed from irreversible inhibitors of Janus kinase (JAK) 3 targeting a cysteine located within the solvent-exposed front region of the ATP binding pocket. Structure-guided design exploiting the differences in the gatekeeper residues enabled the achievement of high selectivity over JAK3 and certain other kinases harboring a sterically demanding residue at this position. The most active compounds inhibited BMX and BTK with apparent IC 50 values in the single digit nanomolar range or below showing moderate selectivity within the TEC family and potent cellular target engagement. These compounds represent an important first step towards selective chemical probes for the protein kinase BMX.
Organizational Affiliation:
Department of Pharmaceutical/Medicinal Chemistry, Institute of Pharmaceutical Sciences, Faculty of Sciences, University of Tübingen, 72076 Tübingen, Germany.