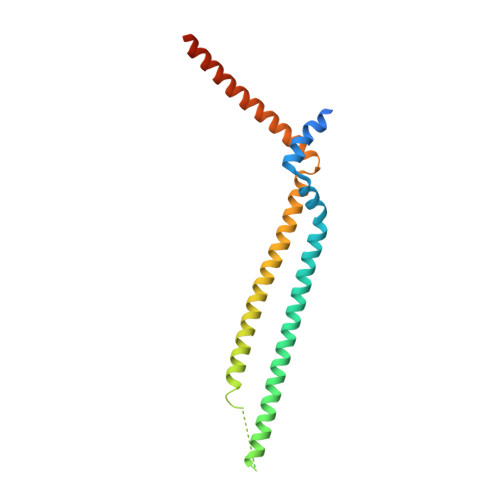
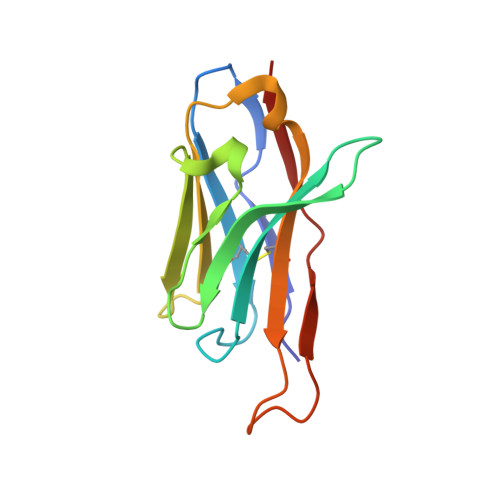
Structure of HIV-1 gp41 with its membrane anchors targeted by neutralizing antibodies.
Caillat, C., Guilligay, D., Torralba, J., Friedrich, N., Nieva, J.L., Trkola, A., Chipot, C.J., Dehez, F.L., Weissenhorn, W.(2021) Elife 10
- PubMed: 33871352
- DOI: https://doi.org/10.7554/eLife.65005
- Primary Citation of Related Structures:
7AEJ - PubMed Abstract:
The HIV-1 gp120/gp41 trimer undergoes a series of conformational changes in order to catalyze gp41-induced fusion of viral and cellular membranes. Here, we present the crystal structure of gp41 locked in a fusion intermediate state by an MPER-specific neutralizing antibody. The structure illustrates the conformational plasticity of the six membrane anchors arranged asymmetrically with the fusion peptides and the transmembrane regions pointing into different directions. Hinge regions located adjacent to the fusion peptide and the transmembrane region facilitate the conformational flexibility that allows high-affinity binding of broadly neutralizing anti-MPER antibodies. Molecular dynamics simulation of the MPER Ab-stabilized gp41 conformation reveals a possible transition pathway into the final post-fusion conformation with the central fusion peptides forming a hydrophobic core with flanking transmembrane regions. This suggests that MPER-specific broadly neutralizing antibodies can block final steps of refolding of the fusion peptide and the transmembrane region, which is required for completing membrane fusion.
Organizational Affiliation:
Univ. Grenoble Alpes, CEA, CNRS, Institut de Biologie Structurale (IBS), Grenoble, France.