The two-domain cyclophilin family protein anaCyp40 of Anabaena sp. regulates photosystem assembly and phycobilisome association
Yadav, S., Schleiff, E., Yildiz, O.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Alr5059 protein | 375 | Nostoc sp. PCC 7120 = FACHB-418 | Mutation(s): 0 Gene Names: alr5059 | 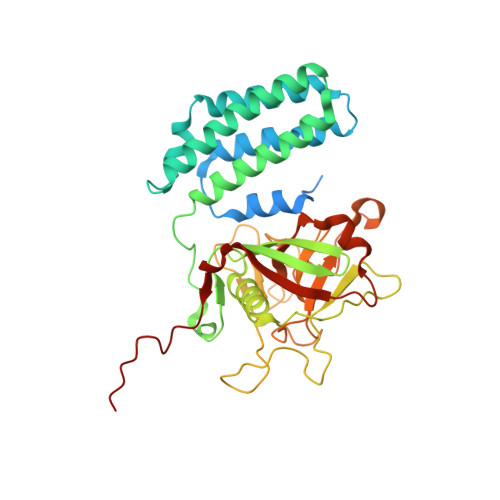 | |
UniProt | |||||
Find proteins for Q8YM80 (Nostoc sp. (strain PCC 7120 / SAG 25.82 / UTEX 2576)) Explore Q8YM80 Go to UniProtKB: Q8YM80 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8YM80 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| CA Query on CA | B [auth A] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 41.12 | α = 90 |
| b = 103.06 | β = 114.74 |
| c = 44.17 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| PHENIX | refinement |
| DIALS | data reduction |
| XSCALE | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| German Research Foundation (DFG) | Germany | DFG SCHL585/7-2) |