Functional, structural, and molecular characterizations of the leukemogenic driver MEF2D-HNRNPUL1 fusion.
Zhang, M., Zhang, H., Li, Z., Bai, L., Wang, Q., Li, J., Jiang, M., Xue, Q., Cheng, N., Zhang, W., Mao, D., Chen, Z., Huang, J., Meng, G., Chen, Z., Chen, S.J.(2022) Blood 140: 1390-1407
- PubMed: 35544603
- DOI: https://doi.org/10.1182/blood.2022016241
- Primary Citation of Related Structures:
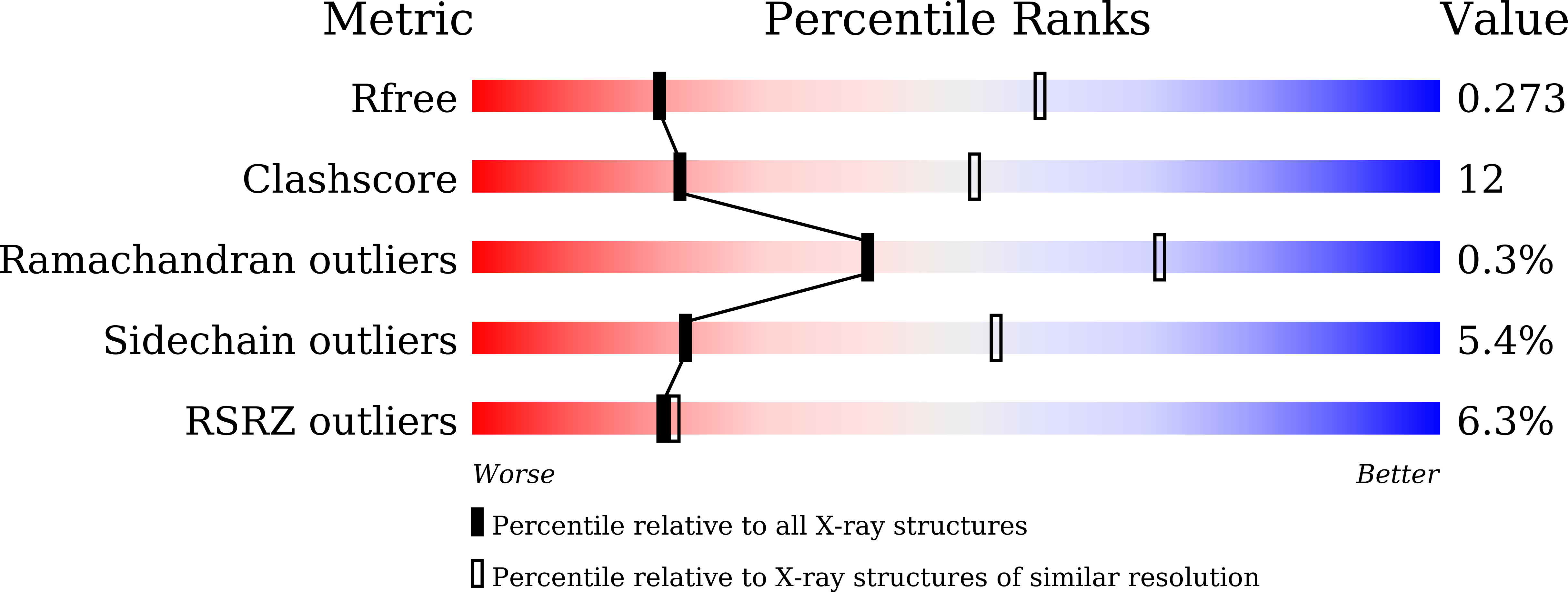
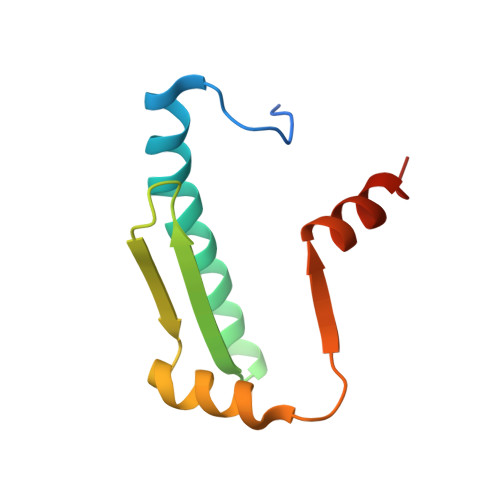
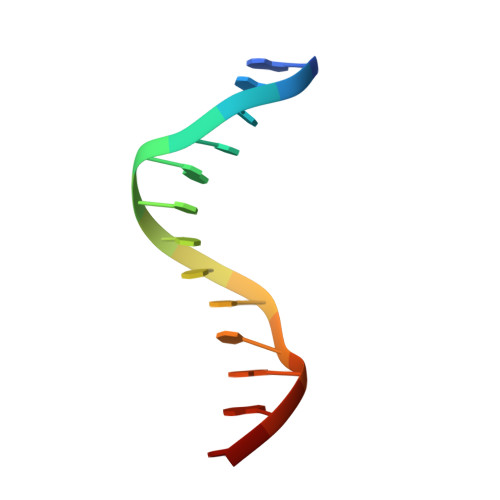
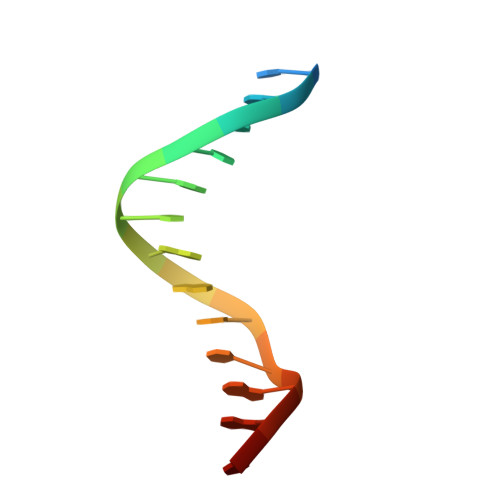
7X1N - PubMed Abstract:
Recurrent MEF2D fusions with poor prognosis have been identified in B-cell precursor ALL (BCP-ALL). The molecular mechanisms underlying the pathogenic function of MEF2D fusions are poorly understood. Here, we show that MEF2D-HNRNPUL1 (MH) knock-in mice developed a progressive disease from impaired B-cell development at the pre-pro-B stage to pre-leukemia over 10 to 12 months. When cooperating with NRASG12D, MH drove an outbreak of BCP-ALL, with a more aggressive phenotype than the NRASG12D-induced leukemia. RNA-sequencing identified key networks involved in disease mechanisms. In chromatin immunoprecipitation-sequencing experiments, MH acquired increased chromatin-binding ability, mostly through MEF2D-responsive element (MRE) motifs in target genes, compared with wild-type MEF2D. Using X-ray crystallography, the MEF2D-MRE complex was characterized in atomic resolution, whereas disrupting the MH-DNA interaction alleviated the aberrant target gene expression and the B-cell differentiation arrest. The C-terminal moiety (HNRNPUL1 part) of MH was proven to contribute to the fusion protein's trans-regulatory activity, cofactor recruitment, and homodimerization. Furthermore, targeting MH-driven transactivation of the HDAC family by using the histone deacetylase inhibitor panobinostat in combination with chemotherapy improved the overall survival of MH/NRASG12D BCP-ALL mice. Altogether, these results not only highlight MH as an important driver in leukemogenesis but also provoke targeted intervention against BCP-ALL with MEF2D fusions.
Organizational Affiliation:
Shanghai Institute of Hematology, State Key Laboratory of Medical Genomics, National Research Center for Translational Medicine at Shanghai, Ruijin Hospital, Shanghai JiaoTong University School of Medicine and School of Life Sciences and Biotechnology, Shanghai JiaoTong University, Shanghai, China.