Engineering of an in-cell protein crystal for fastening a metastable conformation of a target miniprotein.
Kojima, M., Abe, S., Furuta, T., Tran, D.P., Hirata, K., Yamashita, K., Hishikawa, Y., Kitao, A., Ueno, T.(2023) Biomater Sci 11: 1350-1357
- PubMed: 36594419
- DOI: https://doi.org/10.1039/d2bm01759h
- Primary Citation of Related Structures:
7WYR - PubMed Abstract:
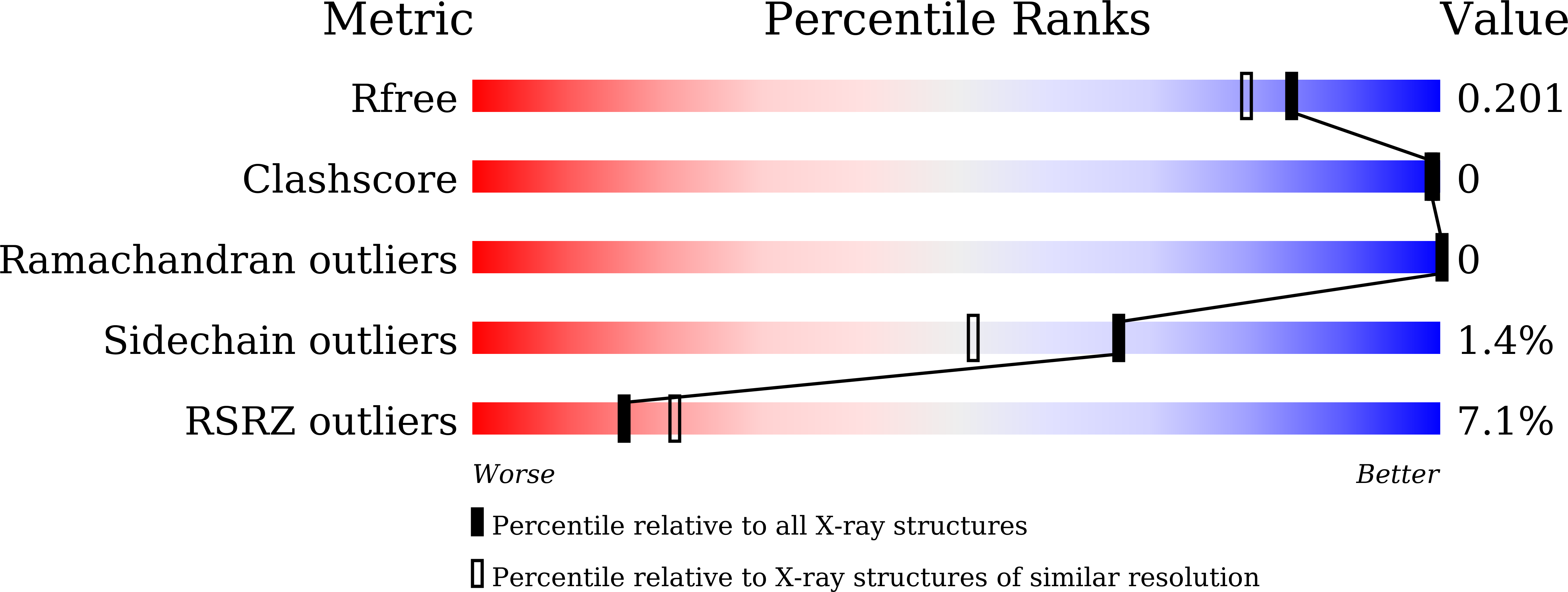
Protein crystals can be utilized as porous scaffolds to capture exogenous molecules. Immobilization of target proteins using protein crystals is expected to facilitate X-ray structure analysis of proteins that are difficult to be crystallized. One of the advantages of scaffold-assisted structure determination is the analysis of metastable structures that are not observed in solution. However, efforts to fix target proteins within the pores of scaffold protein crystals have been limited due to the lack of strategies to control protein-protein interactions formed in the crystals. In this study, we analyze the metastable structure of the miniprotein, CLN025, which forms a β-hairpin structure in solution, using a polyhedra crystal (PhC), an in-cell protein crystal. CLN025 is successfully fixed within the PhC scaffold by replacing the original loop region. X-ray crystal structure analysis and molecular dynamics (MD) simulation reveal that CLN025 is fixed as a helical structure in a metastable state by non-covalent interactions in the scaffold crystal. These results indicate that modulation of intermolecular interactions can trap various protein conformations in the engineered PhC and provides a new strategy for scaffold-assisted structure determination.
Organizational Affiliation:
School of Life Science and Technology, Tokyo Institute of Technology, Nagatsuta-cho 4259, Midori-ku, Yokohama 226-8501, Japan. tueno@bio.titech.ac.jp.