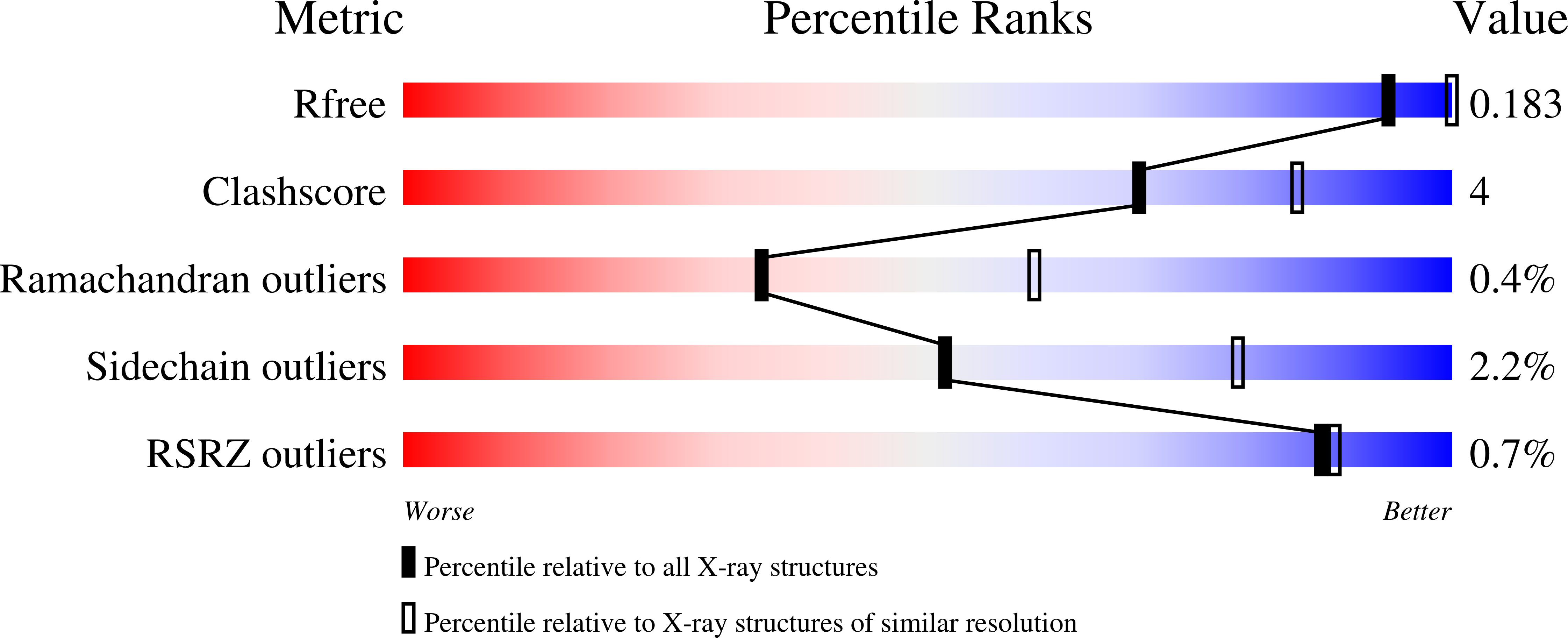
The uncharacterized Pseudomonas aeruginosa PA4189 is a novel and efficient aminoacetaldehyde dehydrogenase.
Fernandez-Silva, A., Juarez-Vazquez, A.L., Gonzalez-Segura, L., Juarez-Diaz, J.A., Munoz-Clares, R.A.(2023) Biochem J 480: 259-281
- PubMed: 36727473
- DOI: https://doi.org/10.1042/BCJ20220567
- Primary Citation of Related Structures:
7UYY - PubMed Abstract:
Neither the Pseudomonas aeruginosa aldehyde dehydrogenase encoded by the PA4189 gene nor its ortholog proteins have been biochemically or structurally characterized and their physiological function is unknown. We cloned the PA4189 gene, obtained the PA4189 recombinant protein, and studied its structure-function relationships. PA4189 is an NAD+-dependent aminoaldehyde dehydrogenase highly efficient with protonated aminoacetaldehyde and 3-aminopropionaldehyde, which are much more preferred to the non-protonated species as indicated by pH studies. Based on the higher activity with aminoacetaldehyde than with 3-aminopropionaldehyde, we propose that aminoacetaldehyde might be the PA4189 physiological substrate. Even though at the physiological pH of P. aeruginosa cells the non-protonated aminoacetaldehyde species will be predominant, and despite the competition of these species with the protonated ones, PA4189 would very efficiently oxidize ACTAL in vivo, producing glycine. To our knowledge, PA4189 is the first reported enzyme that might metabolize ACTAL, which is considered a dead-end metabolite because its consuming reactions are unknown. The PA4189 crystal structure reported here suggested that the charge and size of the active-site residue Glu457, which narrows the aldehyde-entrance tunnel, greatly define the specificity for small positively charged aldehydes, as confirmed by the kinetics of the E457G and E457Q variants. Glu457 and the residues that determine Glu457 conformation inside the active site are conserved in the PA4189 orthologs, which we only found in proteobacteria species. Also is conserved the PA4189 genomic neighborhood, which suggests that PA4189 participates in an uncharacterized metabolic pathway. Our results open the door to future efforts to characterize this pathway.
Organizational Affiliation:
Departamento de Bioquímica, Facultad de Química, Universidad Nacional Autónoma de México, Ciudad de México 04510, Mexico.