Neutralizing epitopes on Clostridioides difficile toxin A revealed by the structures of two camelid VHH antibodies.
Chen, B., Perry, K., Jin, R.(2022) Front Immunol 13: 978858-978858
- PubMed: 36466927
- DOI: https://doi.org/10.3389/fimmu.2022.978858
- Primary Citation of Related Structures:
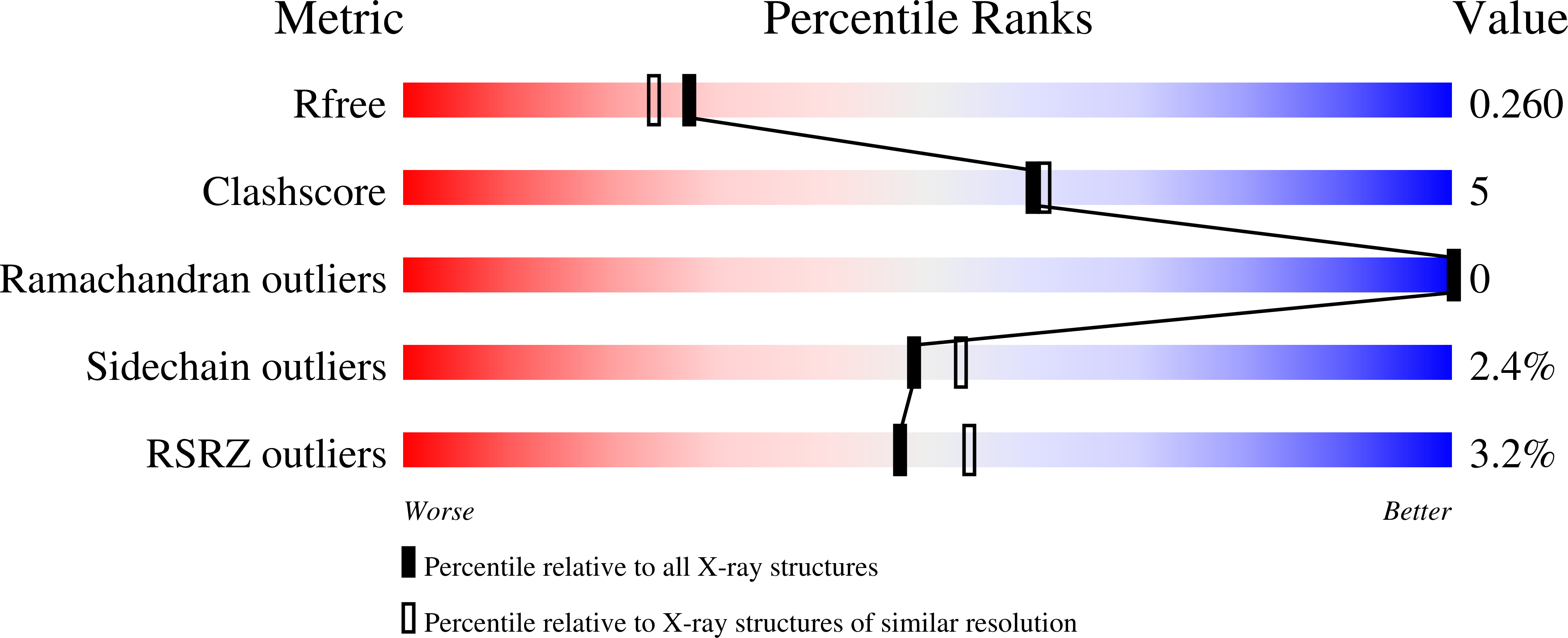
7UBX, 7UBY - PubMed Abstract:
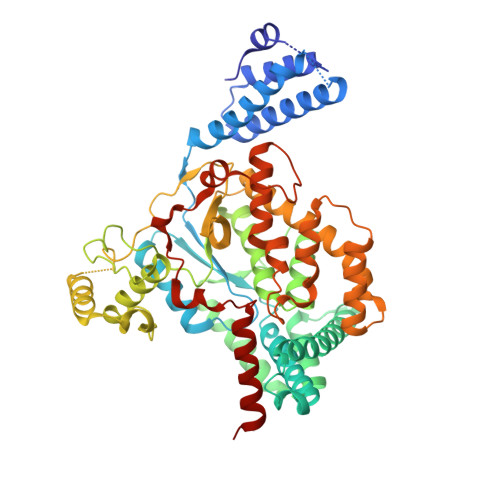
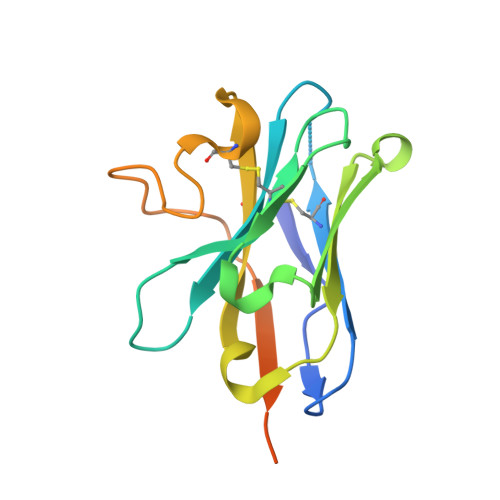
Toxin A (TcdA) and toxin B (TcdB) are two key virulence factors secreted by Clostridioides difficile , which is listed as an urgent threat by the CDC. These two large homologous exotoxins are mainly responsible for diseases associated with C. difficile infection (CDI) with symptoms ranging from diarrhea to life threatening pseudomembranous colitis. Single-domain camelid antibodies (VHHs) AH3 and AA6 are two potent antitoxins against TcdA, which when combined with two TcdB-targeting VHHs showed effective protection against both primary and recurrent CDI in animal models. Here, we report the co-crystal structures of AH3 and AA6 when they form complexes with the glucosyltransferase domain (GTD) and a fragment of the delivery and receptor-binding domain (DRBD) of TcdA, respectively. Based on these structures, we find that AH3 binding enhances the overall stability of the GTD and interferes with its unfolding at acidic pH, and AA6 may inhibit the pH-dependent conformational changes in the DRBD that is necessary for pore formation of TcdA. These studies reveal two functionally critical epitopes on TcdA and shed new insights into neutralizing mechanisms and potential development of epitope-focused vaccines against TcdA.
Organizational Affiliation:
Department of Physiology and Biophysics, School of Medicine, University of California, Irvine, CA, United States.