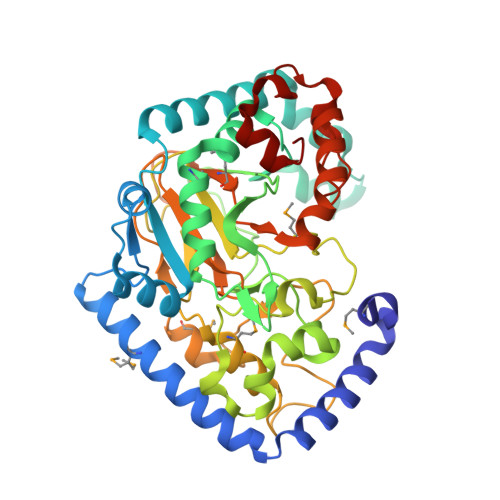
Crystal Structure of the Putative Oxidoreductase of DUF1479-containing Protein Family YPO2976 from Yersinia pestis Bound to CAPS
Kim, Y., Chhor, G., Endres, M., Babnigg, G., Schneewind, O., Joachimiak, A., Center for Structural Genomics of Infectious Diseases (CSGID)To be published.