Expression and Crystallization of HDAC6 Tandem Catalytic Domains.
Langousis, G., Sanchez, J., Kempf, G., Matthias, P.(2023) Methods Mol Biol 2589: 467-480
- PubMed: 36255643
- DOI: https://doi.org/10.1007/978-1-0716-2788-4_30
- Primary Citation of Related Structures:
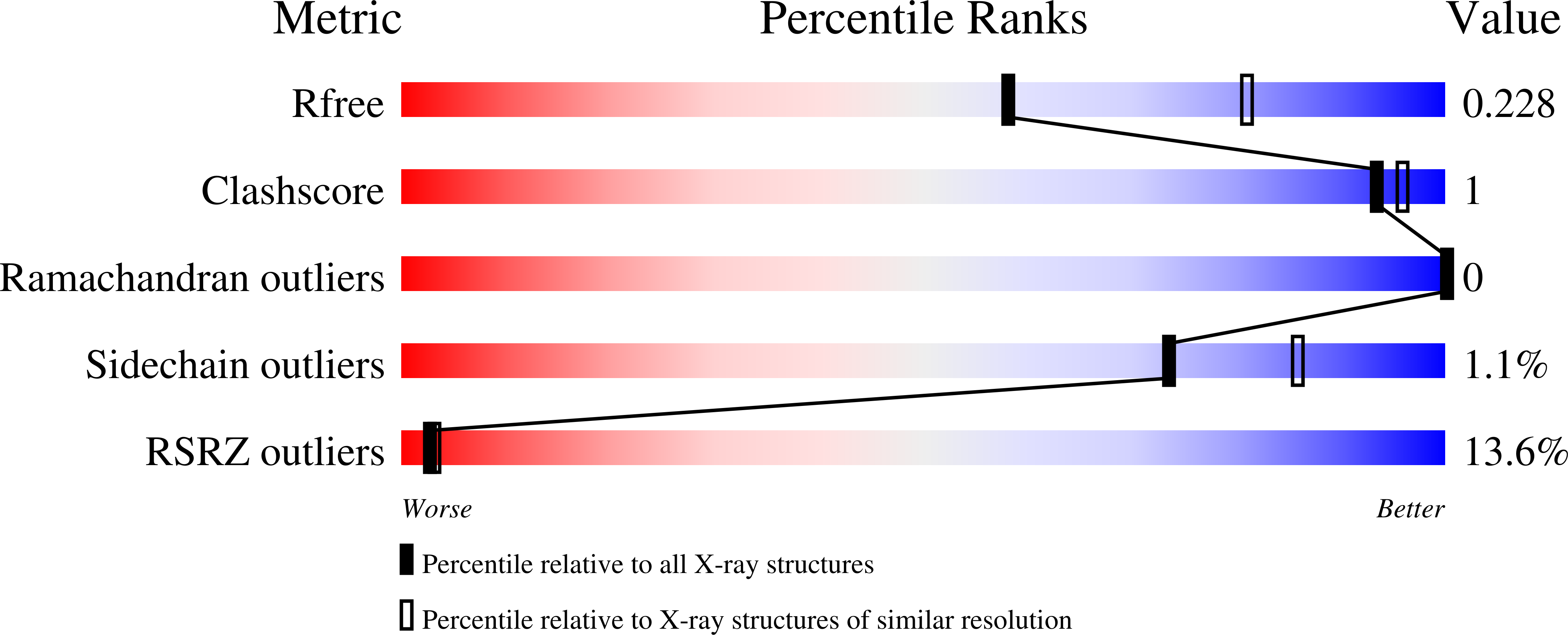
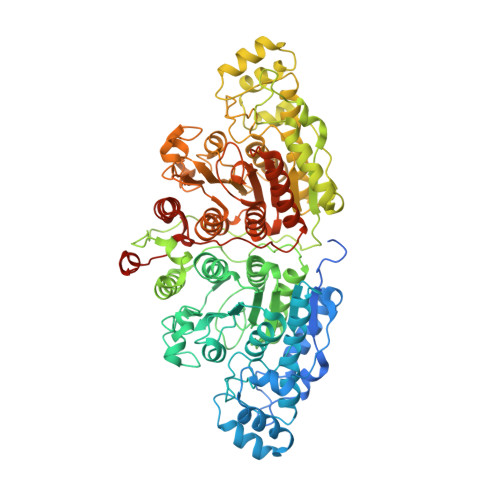
7QNO - PubMed Abstract:
Histone deacetylase 6 (HDAC6) is an atypical lysine deacetylase with tandem catalytic domains and an ubiquitin-binding zinc finger domain. HDAC6 is involved in various biological processes, such as cell motility or stress responses, and has been implicated in pathologies ranging from cancer to neurodegeneration. Due to this broad range of functions, there has been considerable interest in developing HDAC6-specific small molecule inhibitors, several of which are already available. The crystal structure of the tandem catalytic domains of zebrafish HDAC6 has revealed an arrangement with twofold symmetry and extensive surface interaction between the catalytic domains. Further dissection of the biochemical properties of HDAC6 and the development of novel inhibitors will benefit from being able to routinely express high-quality protein. We present here our optimized protocol for expression and crystallization of the zebrafish tandem catalytic domains.
Organizational Affiliation:
Friedrich Miescher Institute for Biomedical Research, Basel, Switzerland.