Towards Translation of PqsR Inverse Agonists: From In Vitro Efficacy Optimization to In Vivo Proof-of-Principle.
Hamed, M.M., Abdelsamie, A.S., Rox, K., Schutz, C., Kany, A.M., Rohrig, T., Schmelz, S., Blankenfeldt, W., Arce-Rodriguez, A., Borrero-de Acuna, J.M., Jahn, D., Rademacher, J., Ringshausen, F.C., Cramer, N., Tummler, B., Hirsch, A.K.H., Hartmann, R.W., Empting, M.(2023) Adv Sci (Weinh) 10: e2204443-e2204443
- PubMed: 36596691
- DOI: https://doi.org/10.1002/advs.202204443
- Primary Citation of Related Structures:
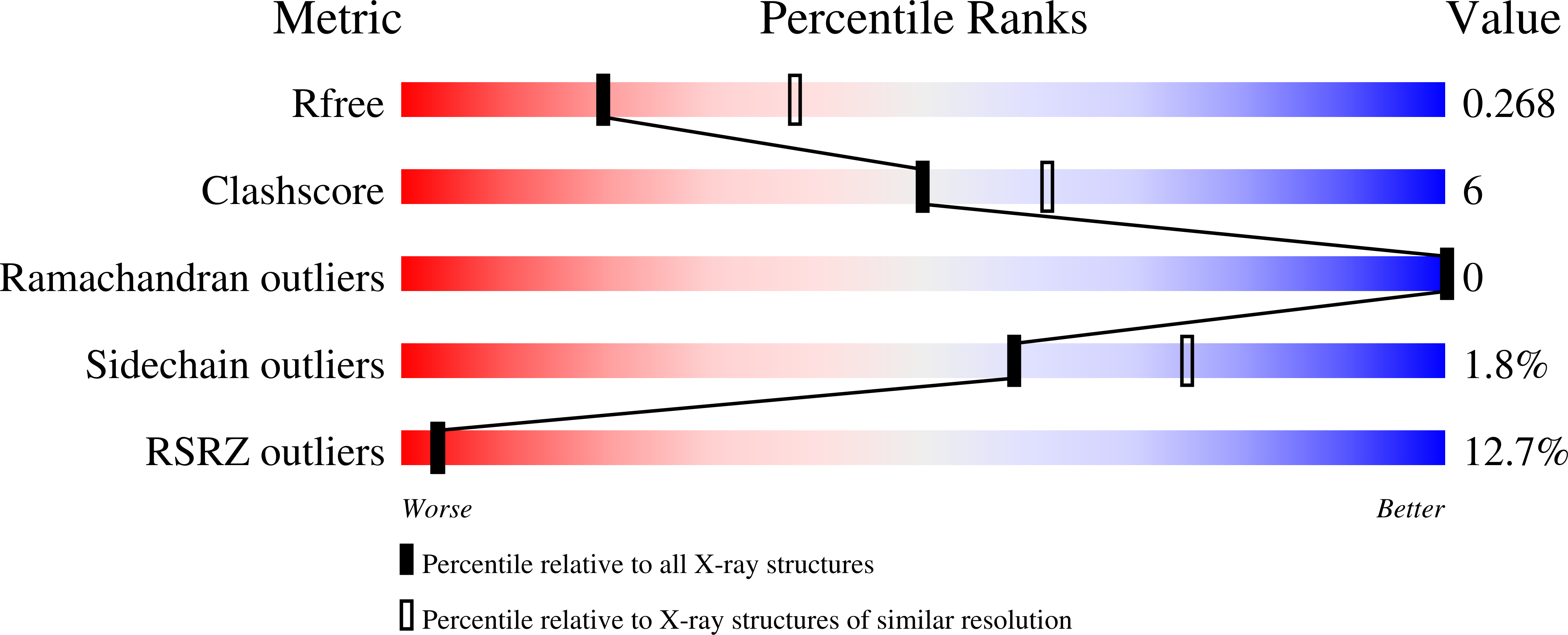
7P4U - PubMed Abstract:
Pseudomonas aeruginosa (PA) is an opportunistic human pathogen, which is involved in a wide range of dangerous infections. It develops alarming resistances toward antibiotic treatment. Therefore, alternative strategies, which suppress pathogenicity or synergize with antibiotic treatments are in great need to combat these infections more effectively. One promising approach is to disarm the bacteria by interfering with their quorum sensing (QS) system, which regulates the release of various virulence factors as well as biofilm formation. Herein, this work reports the rational design, optimization, and in-depth profiling of a new class of Pseudomonas quinolone signaling receptor (PqsR) inverse agonists. The resulting frontrunner compound features a pyrimidine-based scaffold, high in vitro and in vivo efficacy, favorable pharmacokinetics as well as clean safety pharmacology characteristics, which provide the basis for potential pulmonary as well as systemic routes of administration. An X-ray crystal structure in complex with PqsR facilitated further structure-guided lead optimization. The compound demonstrates potent pyocyanin suppression, synergizes with aminoglycoside antibiotic tobramycin against PA biofilms, and is active against a panel of clinical isolates from bronchiectasis patients. Importantly, this in vitro effect translated into in vivo efficacy in a neutropenic thigh infection model in mice providing a proof-of-principle for adjunctive treatment scenarios.
Organizational Affiliation:
Helmholtz-Institute for Pharmaceutical Research Saarland (HIPS), Helmholtz Centre for Infection Research (HZI) Campus E8.1, 66123, Saarbrücken, Germany.