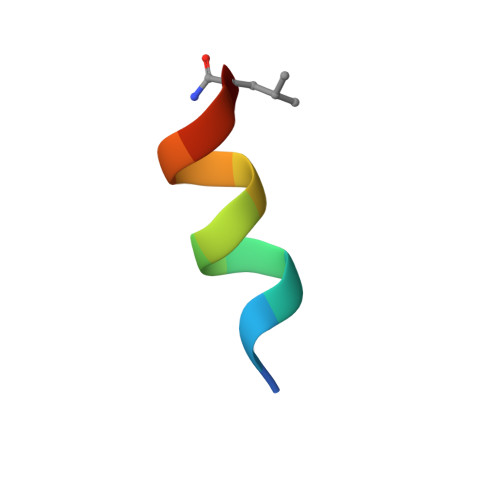A mixed chirality alpha-helix in a stapled bicyclic and a linear antimicrobial peptide revealed by X-ray crystallography.
Baeriswyl, S., Personne, H., Di Bonaventura, I., Kohler, T., van Delden, C., Stocker, A., Javor, S., Reymond, J.L.(2021) RSC Chem Biol 2: 1608-1617
- PubMed: 34977576
- DOI: https://doi.org/10.1039/d1cb00124h
- Primary Citation of Related Structures:
6Y0U, 6Y0V, 6Y13, 6Y14, 6Y1S, 7NEF, 7NEW - PubMed Abstract:
The peptide α-helix is right-handed when containing amino acids with l-chirality, and left-handed with d-chirality, however mixed chirality peptides generally do not form α-helices unless a helix inducer such as the non-natural residue amino-isobutyric acid is used. Herein we report the first X-ray crystal structures of mixed chirality α-helices in short peptides comprising only natural residues as the example of a stapled bicyclic and a linear membrane disruptive amphiphilic antimicrobial peptide (AMP) containing seven l- and four d-residues, as complexes of fucosylated analogs with the bacterial lectin LecB. The mixed chirality α-helices are superimposable onto the homochiral α-helices and form under similar conditions as shown by CD spectra and MD simulations but non-hemolytic and resistant to proteolysis. The observation of a mixed chirality α-helix with only natural residues in the protein environment of LecB suggests a vast unexplored territory of α-helical mixed chirality sequences and their possible use for optimizing bioactive α-helical peptides.
Organizational Affiliation:
Department of Chemistry, Biochemistry and Pharmaceutical Sciences, University of Bern Freiestrasse 3 3012 Bern Switzerland jean-louis.reymond@unibe.ch.