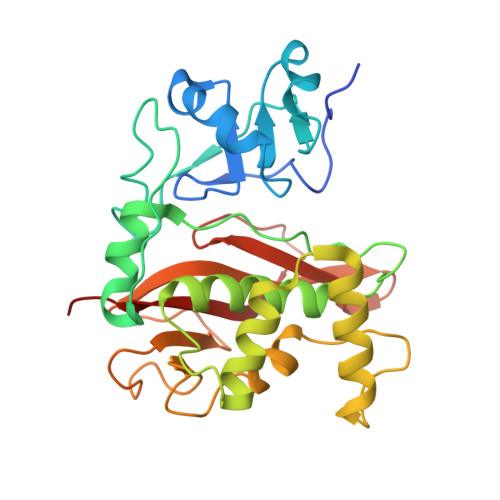
Crystal structure of the Rubella virus protease reveals a unique papain-like protease fold.
Cheong, E.Z.K., Quek, J.P., Xin, L., Li, C., Chan, J.Y., Liew, C.W., Mu, Y., Zheng, J., Luo, D.(2022) J Biol Chem 298: 102250-102250
- PubMed: 35835220
- DOI: https://doi.org/10.1016/j.jbc.2022.102250
- Primary Citation of Related Structures:
7FAV - PubMed Abstract:
Rubella, a viral disease characterized by a red skin rash, is well controlled because of an effective vaccine, but outbreaks are still occurring in the absence of available antiviral treatments. The Rubella virus (RUBV) papain-like protease (RubPro) is crucial for RUBV replication, cleaving the nonstructural polyprotein p200 into two multifunctional proteins, p150 and p90. This protease could represent a potential drug target, but structural and mechanistic details important for the inhibition of this enzyme are unclear. Here, we report a novel crystal structure of RubPro at a resolution of 1.64 Å. The RubPro adopts a unique papain-like protease fold, with a similar catalytic core to that of proteases from Severe acute respiratory syndrome coronavirus 2 and foot-and-mouth disease virus while having a distinctive N-terminal fingers domain. RubPro has well-conserved sequence motifs that are also found in its newly discovered Rubivirus relatives. In addition, we show that the RubPro construct has protease activity in trans against a construct of RUBV protease-helicase and fluorogenic peptides. A protease-helicase construct, exogenously expressed in Escherichia coli, was also cleaved at the p150-p90 cleavage junction, demonstrating protease activity of the protease-helicase protein. We also demonstrate that RubPro possesses deubiquitylation activity, suggesting a potential role of RubPro in modulating the host's innate immune responses. We anticipate that these structural and functional insights of RubPro will advance our current understanding of its function and help facilitate more structure-based research into the RUBV replication machinery, in hopes of developing antiviral therapeutics against RUBV.
Organizational Affiliation:
Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore; School of Biological Sciences, Nanyang Technological University, Singapore, Singapore.