Structures of the human cholecystokinin receptors bound to agonists and antagonists.
Zhang, X., He, C., Wang, M., Zhou, Q., Yang, D., Zhu, Y., Feng, W., Zhang, H., Dai, A., Chu, X., Wang, J., Yang, Z., Jiang, Y., Sensfuss, U., Tan, Q., Han, S., Reedtz-Runge, S., Xu, H.E., Zhao, S., Wang, M.W., Wu, B., Zhao, Q.(2021) Nat Chem Biol 17: 1230-1237
- PubMed: 34556863
- DOI: https://doi.org/10.1038/s41589-021-00866-8
- Primary Citation of Related Structures:
7F8U, 7F8V, 7F8W, 7F8X, 7F8Y - PubMed Abstract:
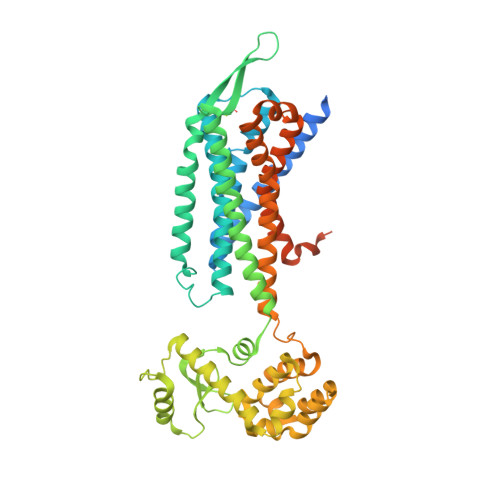
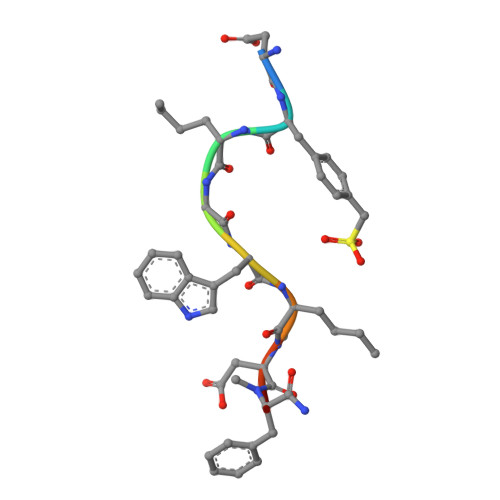
Cholecystokinin receptors, CCK A R and CCK B R, are important neurointestinal peptide hormone receptors and play a vital role in food intake and appetite regulation. Here, we report three crystal structures of the human CCK A R in complex with different ligands, including one peptide agonist and two small-molecule antagonists, as well as two cryo-electron microscopy structures of CCK B R-gastrin in complex with G i2 and G q , respectively. These structures reveal the recognition pattern of different ligand types and the molecular basis of peptide selectivity in the cholecystokinin receptor family. By comparing receptor structures in different conformational states, a stepwise activation process of cholecystokinin receptors is proposed. Combined with pharmacological data, our results provide atomic details for differential ligand recognition and receptor activation mechanisms. These insights will facilitate the discovery of potential therapeutics targeting cholecystokinin receptors.
Organizational Affiliation:
State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China.