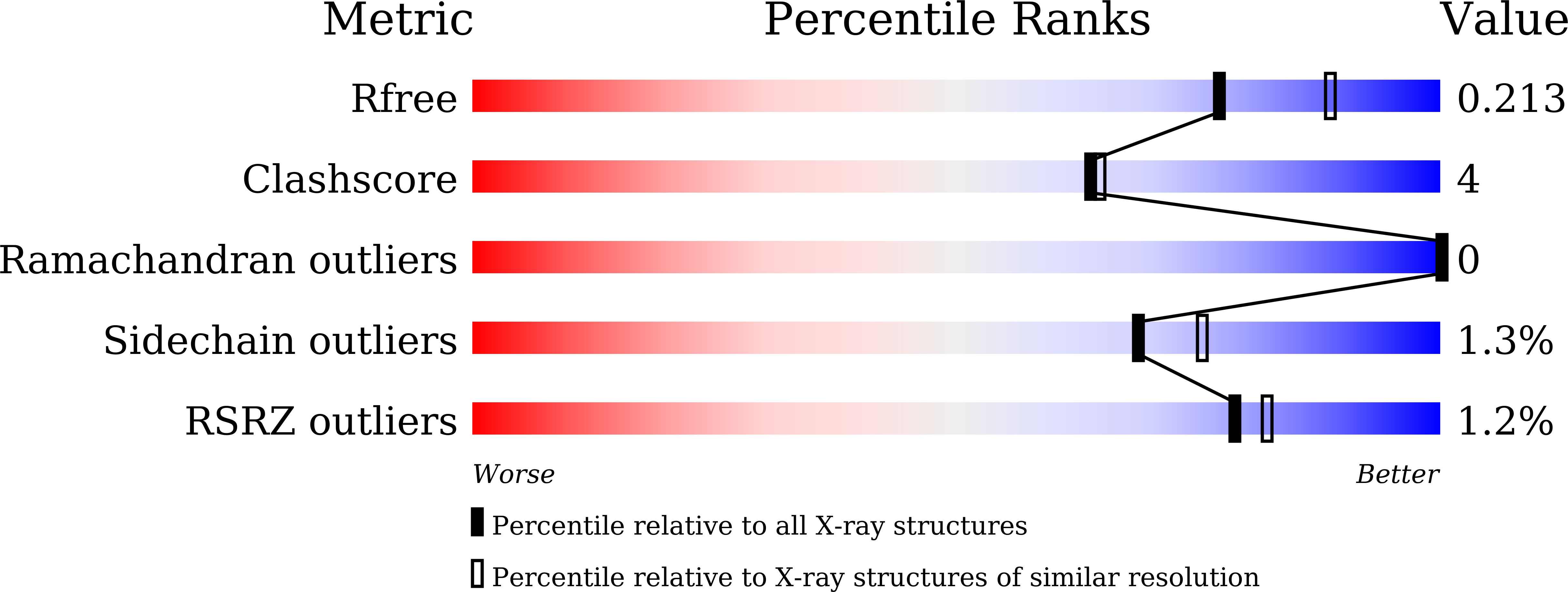
Structure of Pseudomonas aeruginosa spermidine dehydrogenase: a polyamine oxidase with a novel heme-binding fold.
Che, S., Liang, Y., Chen, Y., Wu, W., Liu, R., Zhang, Q., Bartlam, M.(2022) FEBS J 289: 1911-1928
- PubMed: 34741591
- DOI: https://doi.org/10.1111/febs.16264
- Primary Citation of Related Structures:
7D9F, 7D9G, 7D9H, 7D9I, 7D9J - PubMed Abstract:
The opportunistic pathogen Pseudomonas aeruginosa can utilize polyamines (including putrescine, cadaverine, 4-aminobutyrate, spermidine, and spermine) as its sole source of carbon and nitrogen. Spermidine dehydrogenase (SpdH) is a component of one of the two polyamine utilization pathways identified in P. aeruginosa, but little is known about its structure and function. Here, we report the first crystal structure of SpdH from P. aeruginosa to 1.85 Å resolution. The resulting core structure confirms that SpdH belongs to the polyamine oxidase (PAO) family with flavin-binding and substrate-binding domains. A unique N-terminal extension wraps around the flavin-binding domain of SpdH and is required for heme binding, placing a heme cofactor in close proximity to the FAD cofactor. Structural and mutational analysis reveals that residues in the putative active site at the re side of the FAD isoalloxazine ring form part of the catalytic machinery. PaSpdH features an unusual active site and lacks the conserved lysine that forms part of a lysine-water-flavin N5 atom interaction in other PAO enzymes characterized to date. Mutational analysis further confirms that heme is required for catalytic activity. This work provides an important starting point for understanding the role of SpdH, which occurs universally in P. aeruginosa strains, in polyamine metabolism.
Organizational Affiliation:
State Key Laboratory of Medicinal Chemical Biology, Nankai International Advanced Research Institute (Shenzhen Futian), College of Life Sciences, Nankai University, Tianjin, China.