Crystal structure of ebselen covalently bound to the main protease (3CLpro/Mpro) of SARS-CoV-2.
Costanzi, E., Demitri, N., Giabbai, B., Storici, P.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Main Protease | 306 | Severe acute respiratory syndrome coronavirus 2 | Mutation(s): 0 Gene Names: rep, 1a-1b EC: 3.4.19.12 (PDB Primary Data), 3.4.22 (PDB Primary Data), 3.4.22.69 (PDB Primary Data), 2.7.7.48 (PDB Primary Data), 3.6.4.12 (PDB Primary Data), 3.6.4.13 (PDB Primary Data), 3.1.13 (PDB Primary Data), 3.1 (PDB Primary Data), 2.1.1 (PDB Primary Data) | 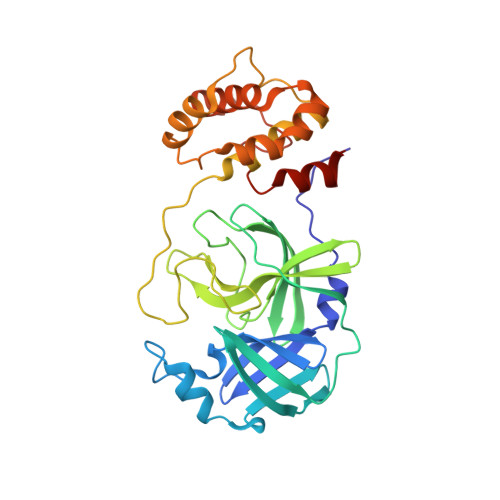 | |
UniProt | |||||
Find proteins for P0DTD1 (Severe acute respiratory syndrome coronavirus 2) Explore P0DTD1 Go to UniProtKB: P0DTD1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0DTD1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| 9JT (Subject of Investigation/LOI) Query on 9JT | C [auth A], D [auth A], E [auth A], L [auth B], O [auth B] | N-phenyl-2-selanylbenzamide C13 H11 N O Se PVPUYGNPKBMXGO-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | F [auth A] G [auth A] H [auth A] I [auth A] K [auth B] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| NA Query on NA | J [auth A], P [auth B] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 67.739 | α = 90 |
| b = 100.455 | β = 90 |
| c = 104.597 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| PHENIX | refinement |
| XDS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| European Commission | European Union | 101003551 |