Structural Insights into the Molecular Recognition Mechanism of the Cancer and Pathogenic Epitope, LacdiNAc by Immune-Related Lectins.
Lima, C.D.L., Coelho, H., Gimeno, A., Trovao, F., Diniz, A., Dias, J.S., Jimenez-Barbero, J., Corzana, F., Carvalho, A.L., Cabrita, E.J., Marcelo, F.(2021) Chemistry 27: 7951-7958
- PubMed: 33826192
- DOI: https://doi.org/10.1002/chem.202100800
- Primary Citation of Related Structures:
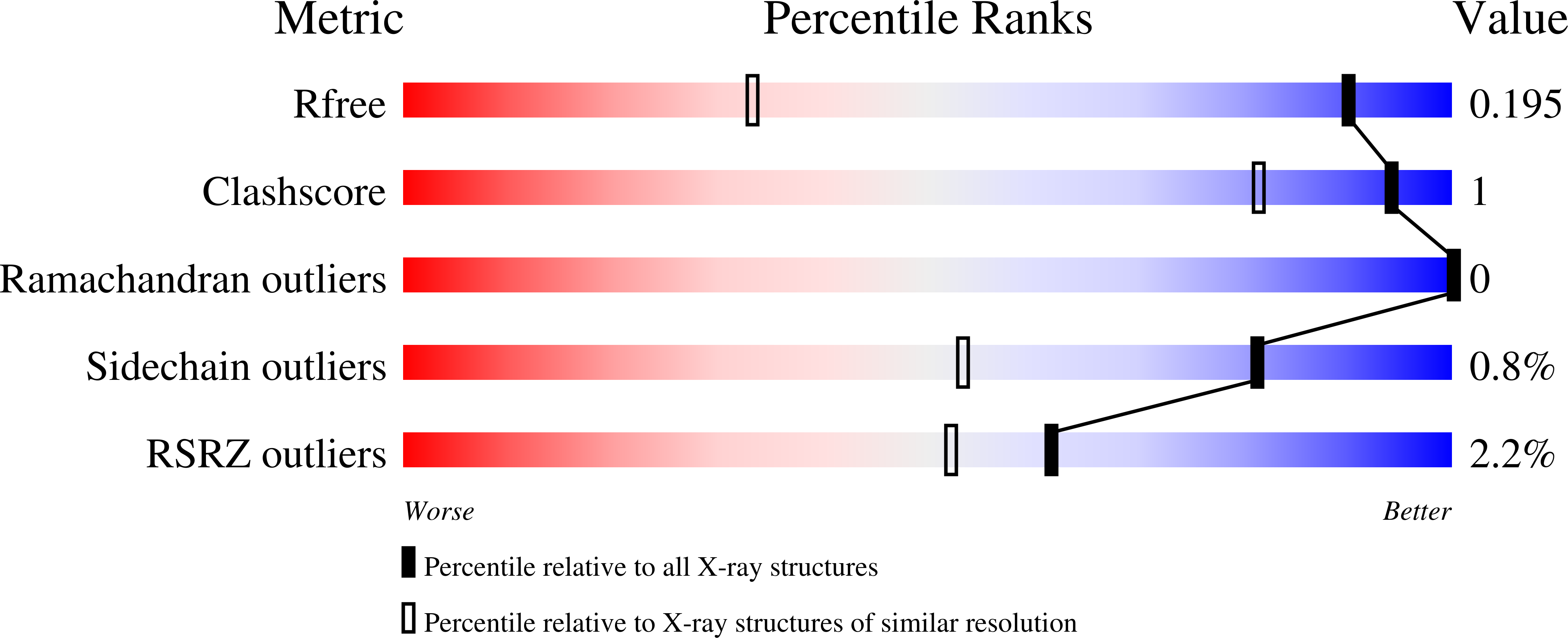
7BE3 - PubMed Abstract:
Interactions of glycan-specific epitopes to human lectin receptors represent novel immune checkpoints for investigating cancer and infection diseases. By employing a multidisciplinary approach that combines isothermal titration calorimetry, NMR spectroscopy, molecular dynamics simulations, and X-ray crystallography, we investigated the molecular determinants that govern the recognition of the tumour and pathogenic glycobiomarker LacdiNAc (GalNAcβ1-4GlcNAc, LDN), including their comparison with the ubiquitous LacNAc epitope (Galβ1-4GlcNAc, LN), by two human immune-related lectins, galectin-3 (hGal-3) and the macrophage galactose C-type lectin (hMGL). A different mechanism of binding and interactions was observed for the hGal-3/LDN and hMGL/LDN complexes, which explains the remarkable difference in the binding specificity of LDN and LN by these two lectins. The new structural clues reported herein are fundamental for the chemical design of mimetics targeting hGal-3/hMGL recognition process.
Organizational Affiliation:
UCIBIO, REQUIMTE, Departamento de Quimica, Faculdade de Ciências e Tecnologia, Universidade NOVA de Lisboa, 2829-516, Caparica, Portugal.