The unique CBM3-Cthe_0271 from Ruminoclostridium thermocellum and CBM3-Clocl_1192 from Hungateiclostridium clariflavum
Milana, M.V., Almog, R., Yaniv, O., Oded, L., Inna, R.G., Felix, F., Edward, A.B., Raphael, L.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cellulose binding domain-containing protein | 148 | Acetivibrio clariflavus DSM 19732 | Mutation(s): 0 Gene Names: Clocl_1192 | 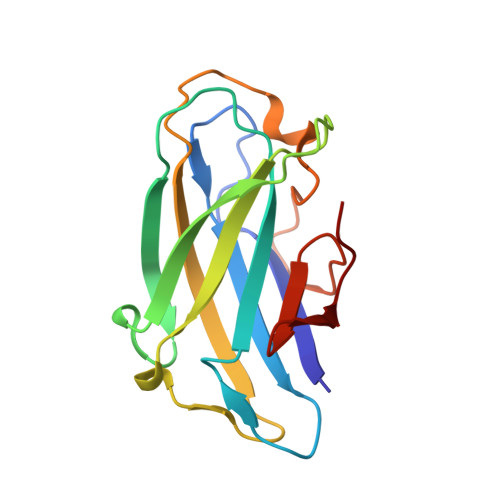 | |
UniProt | |||||
Find proteins for G8LYV1 (Acetivibrio clariflavus (strain DSM 19732 / NBRC 101661 / EBR45)) Explore G8LYV1 Go to UniProtKB: G8LYV1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | G8LYV1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cellulose binding domain-containing protein | 150 | Acetivibrio clariflavus DSM 19732 | Mutation(s): 0 Gene Names: Clocl_1192 | 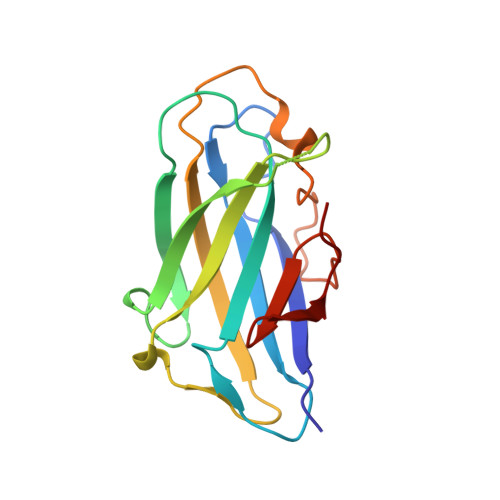 | |
UniProt | |||||
Find proteins for G8LYV1 (Acetivibrio clariflavus (strain DSM 19732 / NBRC 101661 / EBR45)) Explore G8LYV1 Go to UniProtKB: G8LYV1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | G8LYV1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 46.09 | α = 90 |
| b = 157.74 | β = 94.41 |
| c = 46.56 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Israel Science Foundation | Israel | 24/11 |