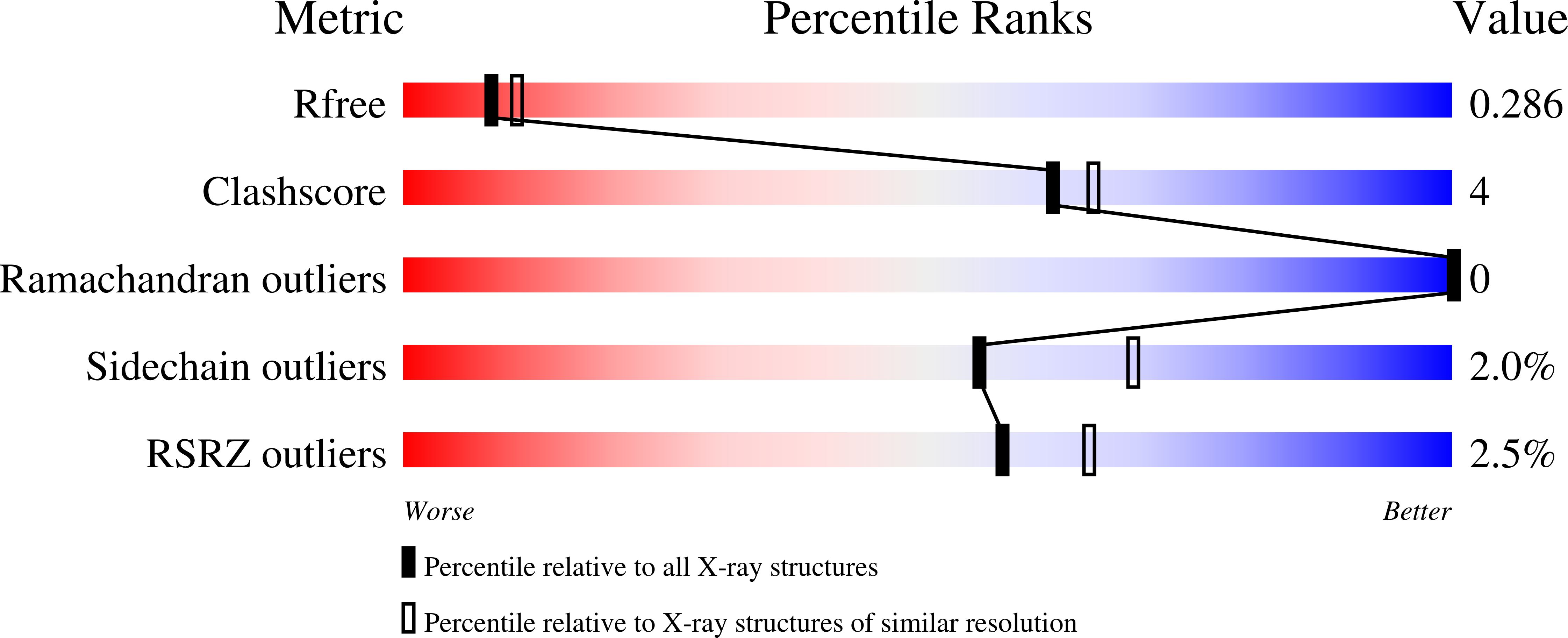
The crystal structure of yeast regulatory subunit reveals key evolutionary insights into Protein Kinase A oligomerization.
Bardeci, N.G., Tofolon, E., Trajtenberg, F., Caramelo, J., Larrieux, N., Rossi, S., Buschiazzo, A., Moreno, S.(2021) J Struct Biol 213: 107732-107732
- PubMed: 33819633
- DOI: https://doi.org/10.1016/j.jsb.2021.107732
- Primary Citation of Related Structures:
6XQK - PubMed Abstract:
Protein Kinase A (PKA) is a widespread enzyme that plays a key role in many signaling pathways from lower eukaryotes to metazoans. In mammals, the regulatory (R) subunits sequester and target the catalytic (C) subunits to proper subcellular locations. This targeting is accomplished by the dimerization and docking (D/D) domain of the R subunits. The activation of the holoenzyme depends on the binding of the second messenger cAMP. The only available structures of the D/D domain proceed from mammalian sources. Unlike dimeric mammalian counterparts, the R subunit from Saccharomyces cerevisiae (Bcy1) forms tetramers in solution. Here we describe the first high-resolution structure of a non-mammalian D/D domain. The tetramer in the crystals of the Bcy1 D/D domain is a dimer of dimers that retain the classical D/D domain fold. By using phylogenetic and structural analyses combined with site-directed mutagenesis, we found that fungal R subunits present an insertion of a single amino acid at the D/D domain that shifts the position of a downstream, conserved arginine. This residue participates in intra-dimer interactions in mammalian D/D domains, while due to this insertion it is involved in inter-dimer contacts in Bcy1, which are crucial for the stability of the tetramer. This surprising finding challenges well-established concepts regarding the oligomeric state within the PKAR protein family and provides important insights into the yet unexplored structural diversity of the D/D domains and the molecular determinants of R subunit oligomerization.
Organizational Affiliation:
Departamento de Química Biológica, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Buenos Aires C1428EHA, Argentina; Instituto de Química Biológica, Facultad de Ciencias Exactas y Naturales (IQUIBICEN-CONICET), Buenos Aires C1428EHA, Argentina. Electronic address: ndgbardeci@gmail.com.