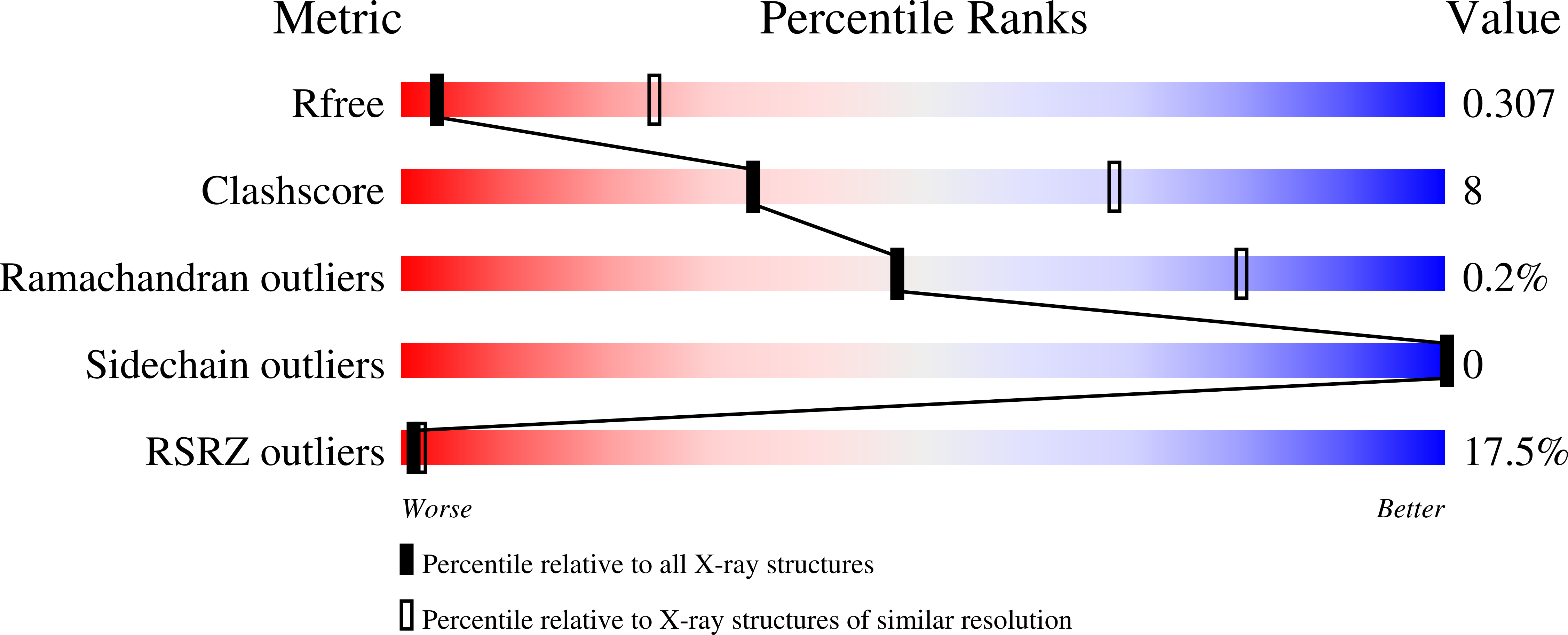
A Prevalent Focused Human Antibody Response to the Influenza Virus Hemagglutinin Head Interface.
McCarthy, K.R., Lee, J., Watanabe, A., Kuraoka, M., Robinson-McCarthy, L.R., Georgiou, G., Kelsoe, G., Harrison, S.C.(2021) mBio 12: e0114421-e0114421
- PubMed: 34060327
- DOI: https://doi.org/10.1128/mBio.01144-21
- Primary Citation of Related Structures:
6XPO, 6XPQ, 6XPR, 6XPX, 6XPY, 6XPZ, 6XQ0, 6XQ2, 6XQ4 - PubMed Abstract:
Novel animal influenza viruses emerge, initiate pandemics, and become endemic seasonal variants that have evolved to escape from prevalent herd immunity. These processes often outpace vaccine-elicited protection. Focusing immune responses on conserved epitopes may impart durable immunity. We describe a focused, protective antibody response, abundant in memory and serum repertoires, to a conserved region at the influenza virus hemagglutinin (HA) head interface. Structures of 11 examples, 8 reported here, from seven human donors demonstrate the convergence of responses on a single epitope. The 11 are genetically diverse, with one class having a common, IGκV1-39, light chain. All of the antibodies bind HAs from multiple serotypes. The lack of apparent genetic restriction and potential for elicitation by more than one serotype may explain their abundance. We define the head interface as a major target of broadly protective antibodies with the potential to influence the outcomes of influenza virus infection. IMPORTANCE The rapid appearance of mutations in circulating human influenza viruses and selection for escape from herd immunity require prediction of likely variants for an annual updating of influenza vaccines. The identification of human antibodies that recognize conserved surfaces on the influenza virus hemagglutinin (HA) has prompted efforts to design immunogens that might selectively elicit such antibodies. The recent discovery of a widely prevalent antibody response to the conserved interface between two HA "heads" (the globular, receptor-binding domains at the apex of the spike-like trimer) has added a new target for these efforts. We report structures of eight such antibodies, bound with HA heads, and compare them with each other and with three others previously described. Although genetically diverse, they all converge on a common binding site. The analysis here can guide immunogen design for preclinical trials.
Organizational Affiliation:
Laboratory of Molecular Medicine, Boston Children's Hospital, Boston, Massachusetts, USA.