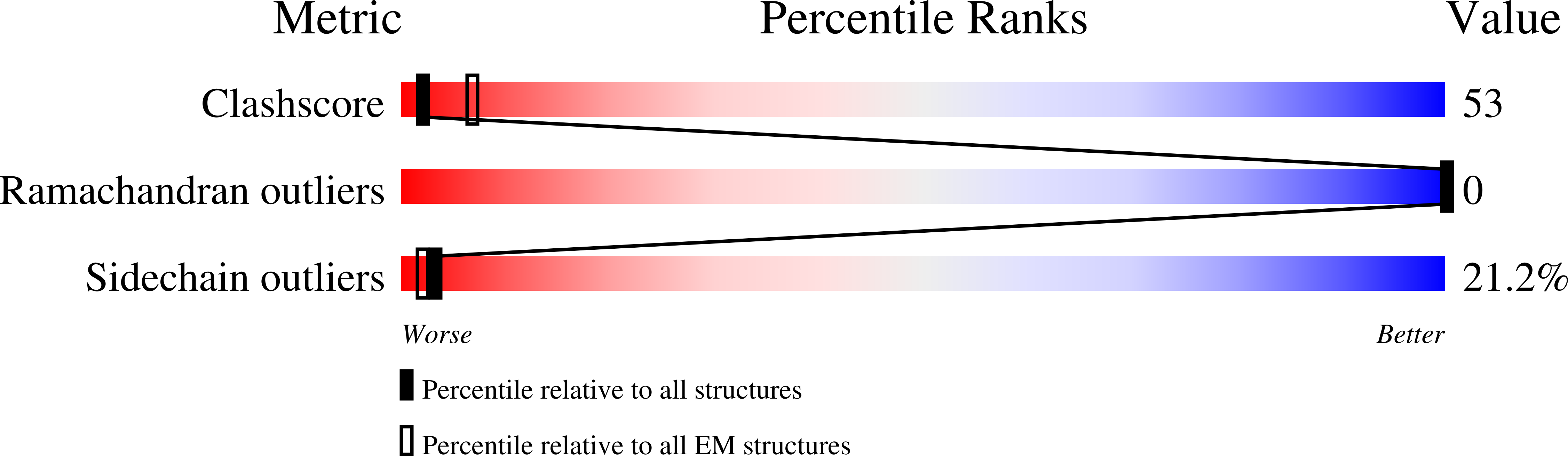Human Antibodies Protect against Aerosolized Eastern Equine Encephalitis Virus Infection.
Williamson, L.E., Gilliland Jr., T., Yadav, P.K., Binshtein, E., Bombardi, R., Kose, N., Nargi, R.S., Sutton, R.E., Durie, C.L., Armstrong, E., Carnahan, R.H., Walker, L.M., Kim, A.S., Fox, J.M., Diamond, M.S., Ohi, M.D., Klimstra, W.B., Crowe Jr., J.E.(2020) Cell 183: 1884-1900.e23
- PubMed: 33301709
- DOI: https://doi.org/10.1016/j.cell.2020.11.011
- Primary Citation of Related Structures:
6XO4, 6XOB - PubMed Abstract:
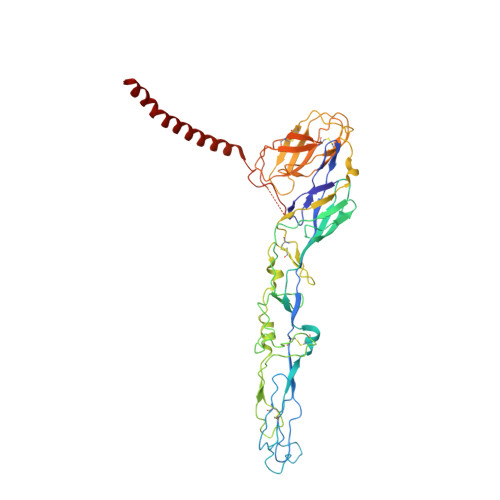
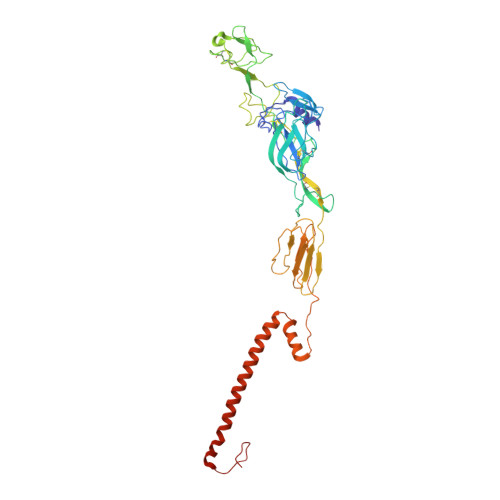
Eastern equine encephalitis virus (EEEV) is one of the most virulent viruses endemic to North America. No licensed vaccines or antiviral therapeutics are available to combat this infection, which has recently shown an increase in human cases. Here, we characterize human monoclonal antibodies (mAbs) isolated from a survivor of natural EEEV infection with potent (<20 pM) inhibitory activity of EEEV. Cryo-electron microscopy reconstructions of two highly neutralizing mAbs, EEEV-33 and EEEV-143, were solved in complex with chimeric Sindbis/EEEV virions to 7.2 Å and 8.3 Å, respectively. The mAbs recognize two distinct antigenic sites that are critical for inhibiting viral entry into cells. EEEV-33 and EEEV-143 protect against disease following stringent lethal aerosol challenge of mice with highly pathogenic EEEV. These studies provide insight into the molecular basis for the neutralizing human antibody response against EEEV and can facilitate development of vaccines and candidate antibody therapeutics.
Organizational Affiliation:
Department of Pathology, Microbiology and Immunology, Vanderbilt University, Nashville, TN 37232, USA.