(p)ppGpp and c-di-AMP Homeostasis Is Controlled by CbpB in Listeria monocytogenes.
Peterson, B.N., Young, M.K.M., Luo, S., Wang, J., Whiteley, A.T., Woodward, J.J., Tong, L., Wang, J.D., Portnoy, D.A.(2020) mBio 11
- PubMed: 32843560
- DOI: https://doi.org/10.1128/mBio.01625-20
- Primary Citation of Related Structures:
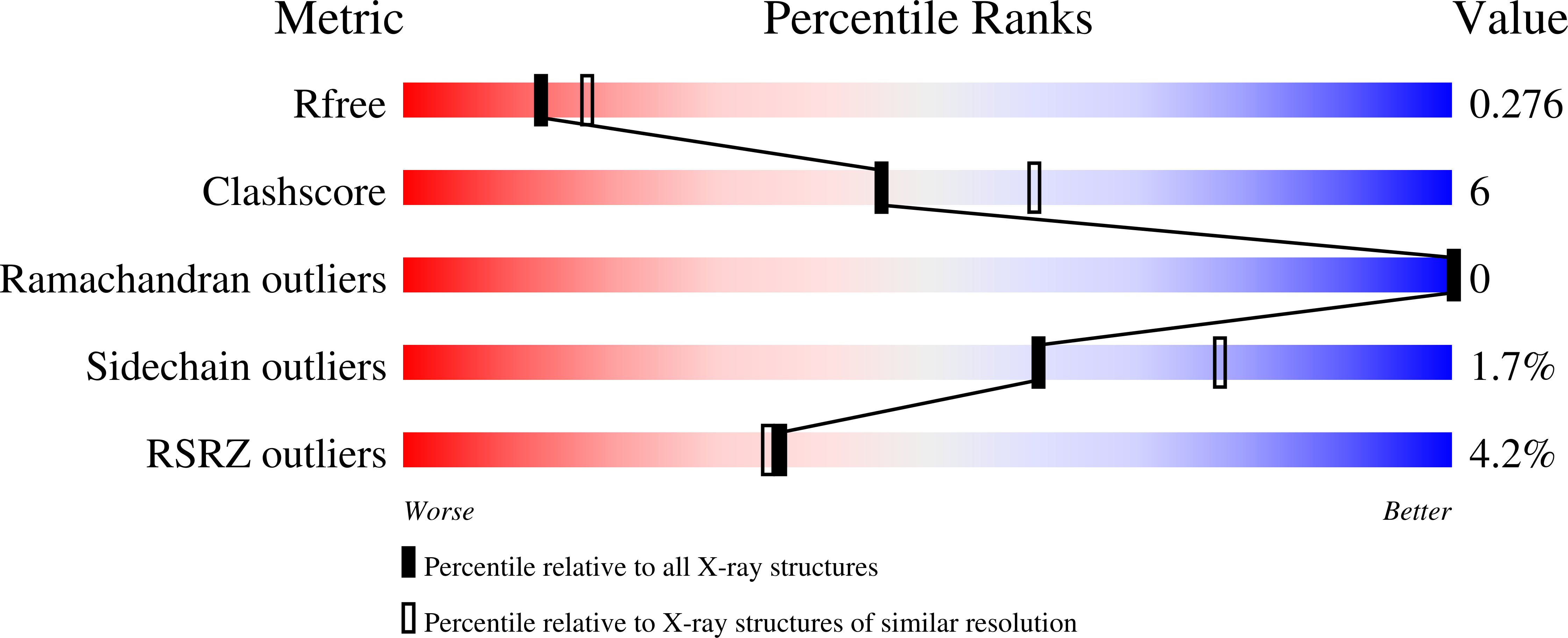
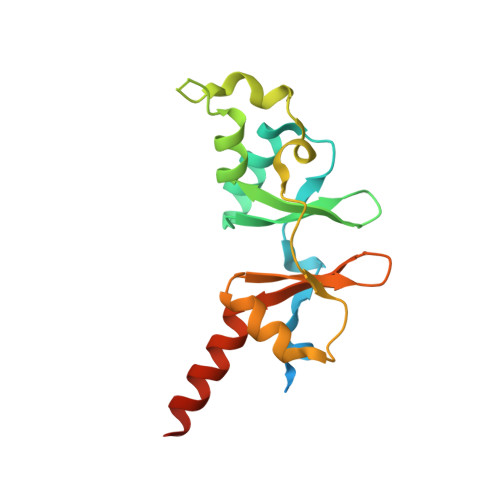
6XNU, 6XNV - PubMed Abstract:
The facultative intracellular pathogen Listeria monocytogenes , like many related Firmicutes , uses the nucleotide second messenger cyclic di-AMP (c-di-AMP) to adapt to changes in nutrient availability, osmotic stress, and the presence of cell wall-acting antibiotics. In rich medium, c-di-AMP is essential; however, mutations in cbpB , the gene encoding c-di-AMP binding protein B, suppress essentiality. In this study, we identified that the reason for cbpB -dependent essentiality is through induction of the stringent response by RelA. RelA is a bifunctional RelA/SpoT homolog (RSH) that modulates levels of (p)ppGpp, a secondary messenger that orchestrates the stringent response through multiple allosteric interactions. We performed a forward genetic suppressor screen on bacteria lacking c-di-AMP to identify genomic mutations that rescued growth while cbpB was constitutively expressed and identified mutations in the synthetase domain of RelA. The synthetase domain of RelA was also identified as an interacting partner of CbpB in a yeast-2-hybrid screen. Biochemical analyses confirmed that free CbpB activates RelA while c-di-AMP inhibits its activation. We solved the crystal structure of CbpB bound and unbound to c-di-AMP and provide insight into the region important for c-di-AMP binding and RelA activation. The results of this study show that CbpB completes a homeostatic regulatory circuit between c-di-AMP and (p)ppGpp in Listeria monocytogenes IMPORTANCE Bacteria must efficiently maintain homeostasis of essential molecules to survive in the environment. We found that the levels of c-di-AMP and (p)ppGpp, two nucleotide second messengers that are highly conserved throughout the microbial world, coexist in a homeostatic loop in the facultative intracellular pathogen Listeria monocytogenes Here, we found that cyclic di-AMP binding protein B (CbpB) acts as a c-di-AMP sensor that promotes the synthesis of (p)ppGpp by binding to RelA when c-di-AMP levels are low. Addition of c-di-AMP prevented RelA activation by binding and sequestering CbpB. Previous studies showed that (p)ppGpp binds and inhibits c-di-AMP phosphodiesterases, resulting in an increase in c-di-AMP. This pathway is controlled via direct enzymatic regulation and indicates an additional mechanism of ribosome-independent stringent activation.
Organizational Affiliation:
Graduate Group in Microbiology, University of California, Berkeley, California, USA.