The Repressor C Protein, Pf4r, Controls Superinfection of Pseudomonas aeruginosa PAO1 by the Pf4 Filamentous Phage and Regulates Host Gene Expression.
Ismail, M.H., Michie, K.A., Goh, Y.F., Noorian, P., Kjelleberg, S., Duggin, I.G., McDougald, D., Rice, S.A.(2021) Viruses 13
- PubMed: 34452479
- DOI: https://doi.org/10.3390/v13081614
- Primary Citation of Related Structures:
6WNM, 6WPZ, 6X6F - PubMed Abstract:
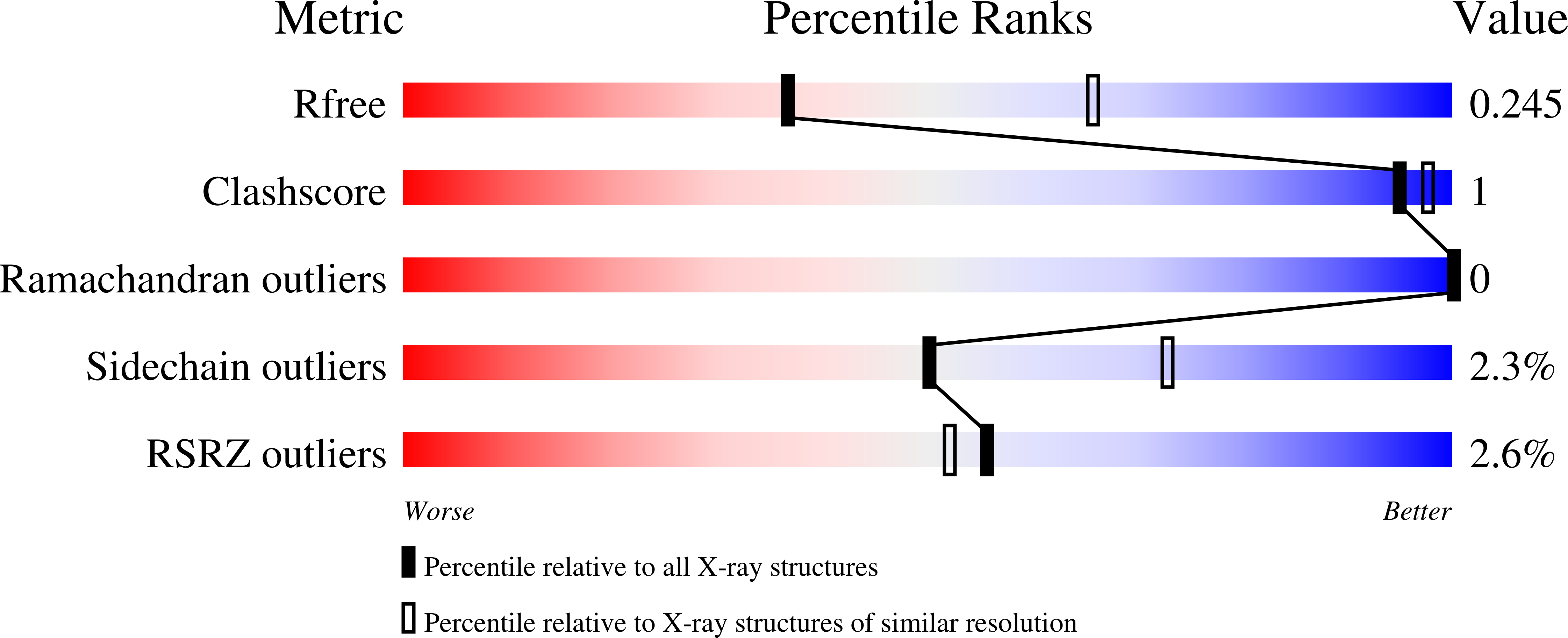
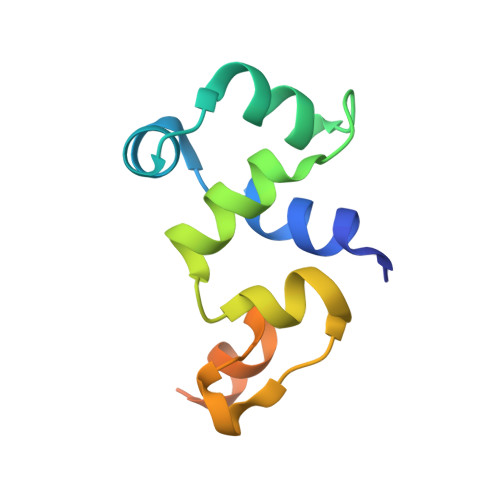
It has been shown that the filamentous phage, Pf4, plays an important role in biofilm development, stress tolerance, genetic variant formation and virulence in Pseudomonas aeruginosa PAO1. These behaviours are linked to the appearance of superinfective phage variants. Here, we have investigated the molecular mechanism of superinfection as well as how the Pf4 phage can control host gene expression to modulate host behaviours. Pf4 exists as a prophage in PAO1 and encodes a homologue of the P2 phage repressor C and was recently named Pf4r. Through a combination of molecular techniques, ChIPseq and transcriptomic analyses, we show a critical site in repressor C (Pf4r) where a mutation in the site, 788799A>G (Ser4Pro), causes Pf4r to lose its function as the immunity factor against reinfection by Pf4. X-ray crystal structure analysis shows that Pf4r forms symmetric homo-dimers homologous to the E.coli bacteriophage P2 RepC protein. A mutation, Pf4r*, associated with the superinfective Pf4r variant, found at the dimer interface, suggests dimer formation may be disrupted, which derepresses phage replication. This is supported by multi-angle light scattering (MALS) analysis, where the Pf4r* protein only forms monomers. The loss of dimerisation also explains the loss of Pf4r's immunity function. Phenotypic assays showed that Pf4r increased LasB activity and was also associated with a slight increase in the percentage of morphotypic variants. ChIPseq and transcriptomic analyses suggest that Pf4r also likely functions as a transcriptional regulator for other host genes. Collectively, these data suggest the mechanism by which filamentous phages play such an important role in P. aeruginosa biofilm development.
Organizational Affiliation:
Singapore Centre for Environmental Life Sciences Engineering, Singapore 637551, Singapore.