Tartryl-CoA inhibits succinyl-CoA synthetase
Huang, J., Fraser, M.E.(2020) Acta Crystallogr F Struct Biol Commun 76: 302
Experimental Data Snapshot
(2020) Acta Crystallogr F Struct Biol Commun 76: 302
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Succinate--CoA ligase [ADP/GDP-forming] subunit alpha, mitochondrial | 315 | Homo sapiens | Mutation(s): 0 Gene Names: SUCLG1 EC: 6.2.1.4 (PDB Primary Data), 6.2.1.5 (PDB Primary Data) | 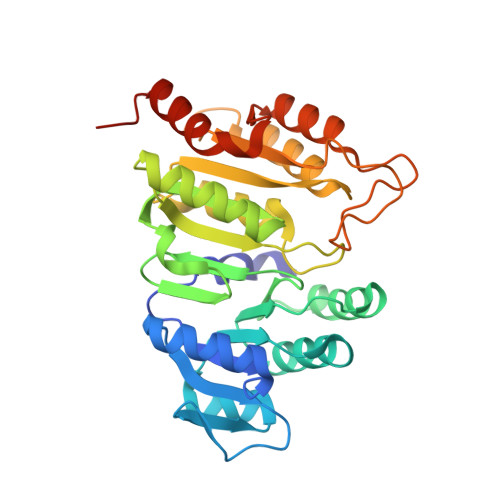 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P53597 (Homo sapiens) Explore P53597 Go to UniProtKB: P53597 | |||||
PHAROS: P53597 GTEx: ENSG00000163541 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P53597 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Succinate--CoA ligase [GDP-forming] subunit beta, mitochondrial | 395 | Homo sapiens | Mutation(s): 0 Gene Names: SUCLG2 EC: 6.2.1.4 | 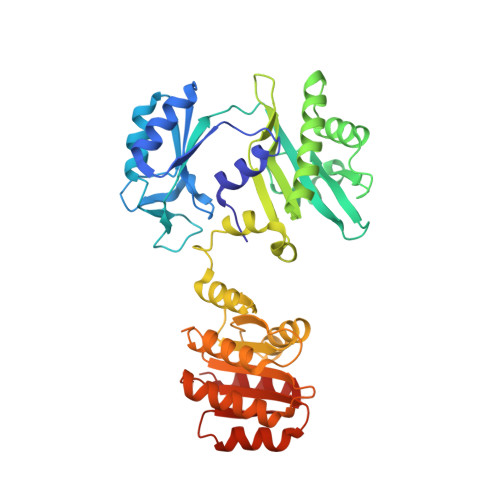 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q96I99 (Homo sapiens) Explore Q96I99 Go to UniProtKB: Q96I99 | |||||
PHAROS: Q96I99 GTEx: ENSG00000172340 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q96I99 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| TUY (Subject of Investigation/LOI) Query on TUY | C [auth A] | (3S,5S,9R,20R,21R)-1-[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)tetrahydrofuran-2-yl]-3,5,9,20,
21-pentahydroxy-8,8-dimethyl-10,14,19-trioxo-2,4,6-trioxa-18-thia-11,15-diaza-3,5-diphosphadocosan-22-oic acid 3,5-dioxide (non-preferred name) C25 H40 N7 O21 P3 S WLCAXJYUSAHDIC-HNBYOPSBSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 87.069 | α = 90 |
| b = 82.465 | β = 102.87 |
| c = 49.276 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| xia2 | data scaling |
| PDB_EXTRACT | data extraction |
| xia2 | data reduction |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Natural Sciences and Engineering Research Council (NSERC, Canada) | Canada | 04815-2019 |