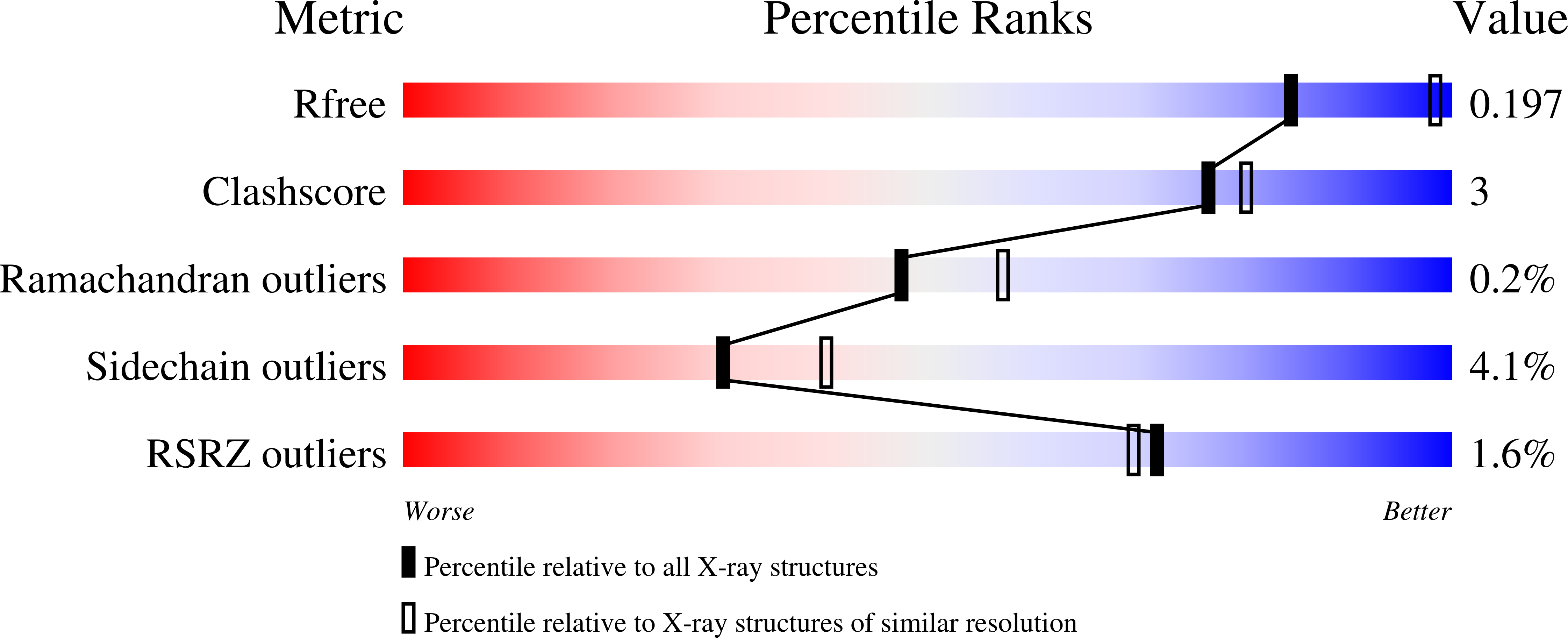
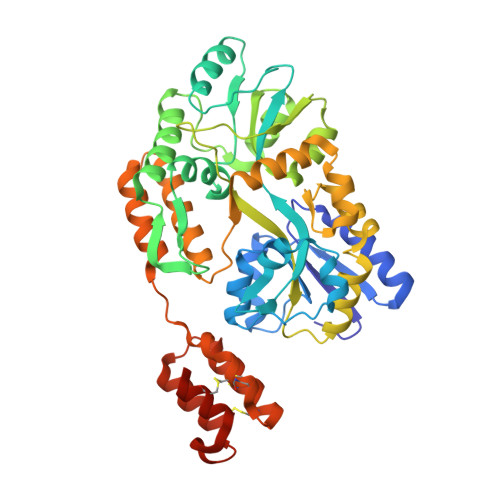
Structure of the RECK CC domain, an evolutionary anomaly.
Chang, T.H., Hsieh, F.L., Smallwood, P.M., Gabelli, S.B., Nathans, J.(2020) Proc Natl Acad Sci U S A 117: 15104-15111
- PubMed: 32541044
- DOI: https://doi.org/10.1073/pnas.2006332117
- Primary Citation of Related Structures:
6WBH, 6WBJ - PubMed Abstract:
Five small protein domains, the CC-domains, at the N terminus of the RECK protein, play essential roles in signaling by WNT7A and WNT7B in the context of central nervous system angiogenesis and blood-brain barrier formation and maintenance. We have determined the structure of CC domain 4 (CC4) at 1.65-Å resolution and find that it folds into a compact four-helix bundle with three disulfide bonds. The CC4 structure, together with homology modeling of CC1, reveals the surface locations of critical residues that were shown in previous mutagenesis studies to mediate GPR124 binding and WNT7A/WNT7B recognition and signaling. Surprisingly, sequence and structural homology searches reveal no other cell-surface or secreted domains in vertebrates that resemble the CC domain, a pattern that is in striking contrast to other ancient and similarly sized domains, such as Epidermal Growth Factor, Fibronectin Type 3, Immunoglobulin, and Thrombospondin type 1 domains, which are collectively present in hundreds of proteins.
Organizational Affiliation:
Department of Molecular Biology and Genetics, Johns Hopkins University School of Medicine, Baltimore, MD 21205.