Comparative Modeling of CDK9 Inhibitors to Explore Selectivity and Structure-Activity Relationships
Kirubakaran, P., Morton, G., Zhang, P., Zhang, H., Abou-Gharbia, M., Issa, J., Wu, J., Childers, W., Karanicolas, J.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cyclin-dependent kinase 9 | 330 | Homo sapiens | Mutation(s): 0 Gene Names: CDK9, CDC2L4, TAK EC: 2.7.11.22 (PDB Primary Data), 2.7.11.23 (PDB Primary Data) | 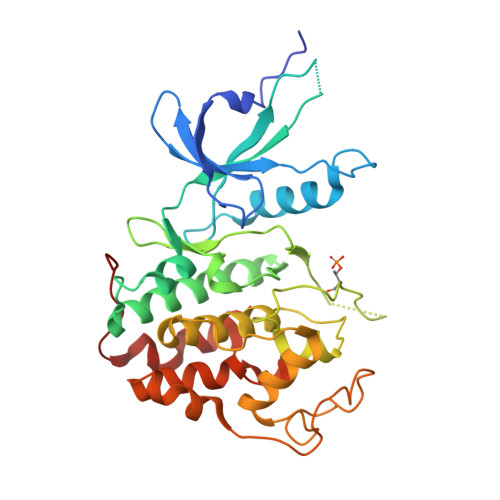 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P50750 (Homo sapiens) Explore P50750 Go to UniProtKB: P50750 | |||||
PHAROS: P50750 GTEx: ENSG00000136807 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P50750 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cyclin-T1 | 259 | Homo sapiens | Mutation(s): 3 Gene Names: CCNT1 | 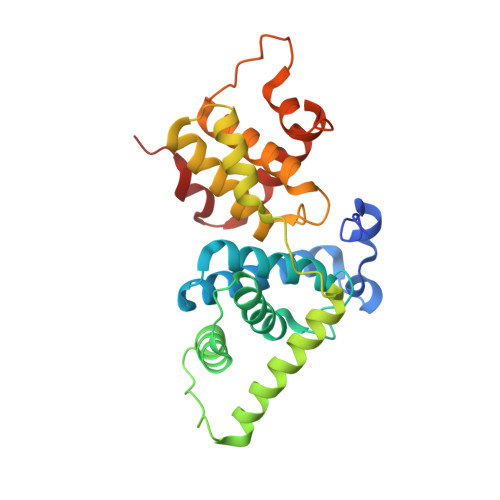 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O60563 (Homo sapiens) Explore O60563 Go to UniProtKB: O60563 | |||||
PHAROS: O60563 GTEx: ENSG00000129315 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O60563 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| TOJ Query on TOJ | E [auth A], F [auth A] | (4-amino-2-{[(1R,2R,4S)-bicyclo[2.2.1]heptan-2-yl]amino}-1,3-thiazol-5-yl)(2-nitrophenyl)methanone C17 H18 N4 O3 S JRNXAQINDCOHGS-HOSYDEDBSA-N |  | ||
| TO7 Query on TO7 | C [auth A], D [auth A] | (4-amino-2-{[(1S,2S,4R)-bicyclo[2.2.1]heptan-2-yl]amino}-1,3-thiazol-5-yl)(2-nitrophenyl)methanone C17 H18 N4 O3 S JRNXAQINDCOHGS-SCVCMEIPSA-N |  | ||
| PO4 Query on PO4 | G [auth B] | PHOSPHATE ION O4 P NBIIXXVUZAFLBC-UHFFFAOYSA-K |  | ||
| Modified Residues 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| SEP Query on SEP | A | L-PEPTIDE LINKING | C3 H8 N O6 P |  | SER |
| TPO Query on TPO | A | L-PEPTIDE LINKING | C4 H10 N O6 P |  | THR |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 173.34 | α = 90 |
| b = 173.34 | β = 90 |
| c = 97.368 | γ = 120 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| XDS | data reduction |
| XDS | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | GM119560 |
| American Cancer Society | United States | RSG-15-167-01-DMC |