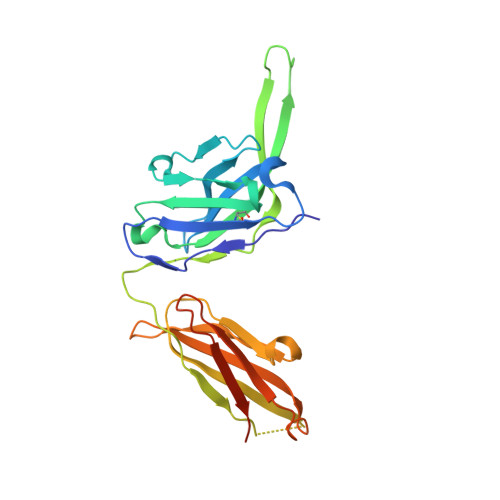
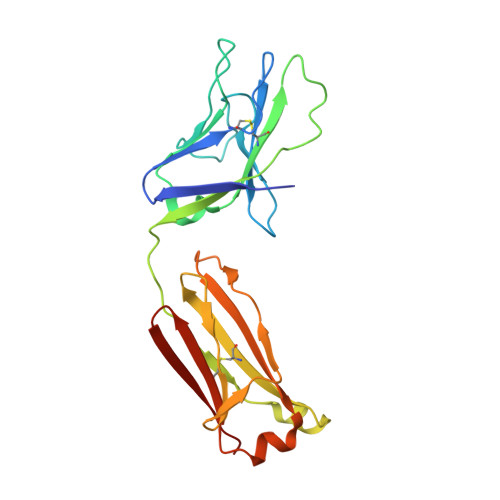
Development of a 3Mut-Apex-Stabilized Envelope Trimer That Expands HIV-1 Neutralization Breadth When Used To Boost Fusion Peptide-Directed Vaccine-Elicited Responses.
Chuang, G.Y., Lai, Y.T., Boyington, J.C., Cheng, C., Geng, H., Narpala, S., Rawi, R., Schmidt, S.D., Tsybovsky, Y., Verardi, R., Xu, K., Yang, Y., Zhang, B., Chambers, M., Changela, A., Corrigan, A.R., Kong, R., Olia, A.S., Ou, L., Sarfo, E.K., Wang, S., Wu, W., Doria-Rose, N.A., McDermott, A.B., Mascola, J.R., Kwong, P.D.(2020) J Virol 94
- PubMed: 32295908
- DOI: https://doi.org/10.1128/JVI.00074-20
- Primary Citation of Related Structures:
6VZI, 6W03 - PubMed Abstract:
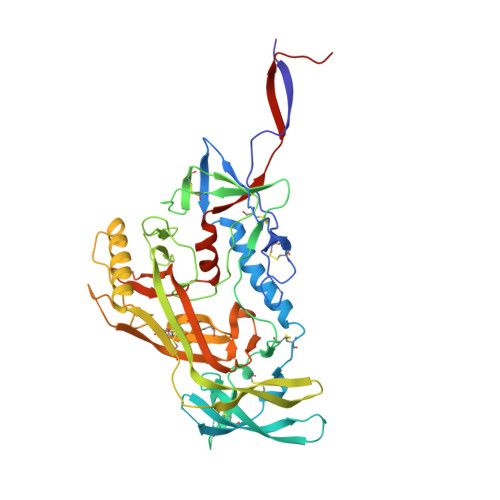
HIV-1 envelope (Env) trimers, stabilized in a prefusion-closed conformation, can elicit humoral responses capable of neutralizing HIV-1 strains closely matched in sequence to the immunizing strain. One strategy to increase elicited neutralization breadth involves vaccine priming of immune responses against a target site of vulnerability, followed by vaccine boosting of these responses with prefusion-closed Env trimers. This strategy has succeeded at the fusion peptide (FP) site of vulnerability in eliciting cross-clade neutralizing responses in standard vaccine-test animals. However, the breadth and potency of the elicited responses have been less than optimal. Here, we identify three mutations (3mut), Met302, Leu320, and Pro329, that stabilize the apex of the Env trimer in a prefusion-closed conformation and show antigenically, structurally, and immunogenically that combining 3mut with other approaches (e.g., repair and stabilize and glycine-helix breaking) yields well-behaved clade C-Env trimers capable of boosting the breadth of FP-directed responses. Crystal structures of these trimers confirmed prefusion-closed apexes stabilized by hydrophobic patches contributed by Met302 and Leu320, with Pro329 assuming canonically restricted dihedral angles. We substituted the N-terminal eight residues of FP (FP8, residues 512 to 519) of these trimers with the second most prevalent FP8 sequence (FP8v2, AVGLGAVF) and observed a 3mut-stabilized consensus clade C-Env trimer with FP8v2 to boost the breadth elicited in guinea pigs of FP-directed responses induced by immunogens containing the most prevalent FP8 sequence (FP8v1, AVGIGAVF). Overall, 3mut can stabilize the Env trimer apex, and the resultant apex-stabilized Env trimers can be used to expand the neutralization breadth elicited against the FP site of vulnerability. IMPORTANCE A major hurdle to the development of an effective HIV-1 vaccine is the elicitation of serum responses capable of neutralizing circulating strains of HIV, which are extraordinarily diverse in sequence and often highly neutralization resistant. Recently, we showed how sera with 20 to 30% neutralization breadth could, nevertheless, be elicited in standard vaccine test animals by priming with the most prevalent N-terminal 8 residues of the HIV-1 fusion peptide (FP8), followed by boosting with a stabilized BG505-envelope (Env) trimer. Here, we show that subsequent boosting with a 3mut-apex-stabilized consensus C-Env trimer, modified to have the second most prevalent FP8 sequence, elicits higher neutralization breadth than that induced by continued boosting with the stabilized BG505-Env trimer. With increased neutralizing breadth elicited by boosting with a heterologous trimer containing the second most prevalent FP8 sequence, the fusion peptide-directed immune-focusing approach moves a step closer toward realizing an effective HIV-1 vaccine regimen.
Organizational Affiliation:
Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.