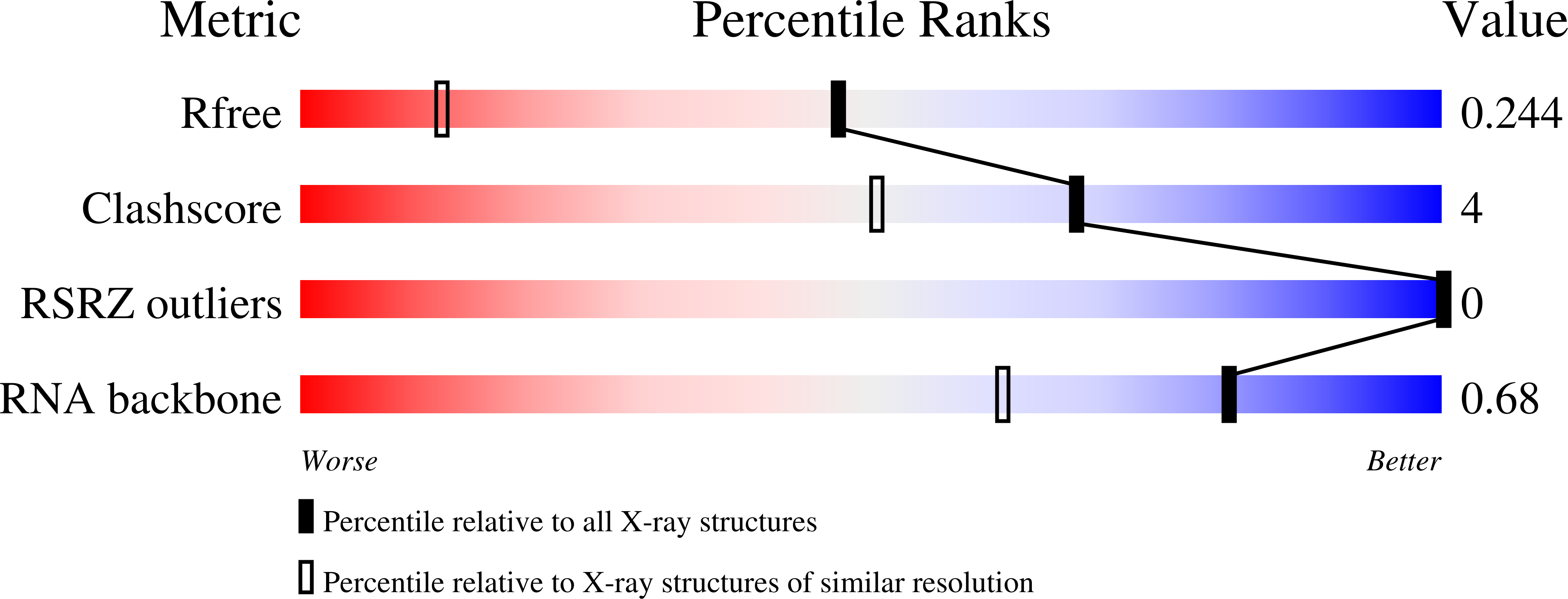Synthesis, chirality-dependent conformational and biological properties of siRNAs containing 5'-(R)- and 5'-(S)-C-methyl-guanosine.
Mikami, A., Erande, N., Matsuda, S., Kel'in, A., Woods, L.B., Chickering, T., Pallan, P.S., Schlegel, M.K., Zlatev, I., Egli, M., Manoharan, M.(2020) Nucleic Acids Res 48: 10101-10124
- PubMed: 32990754
- DOI: https://doi.org/10.1093/nar/gkaa750
- Primary Citation of Related Structures:
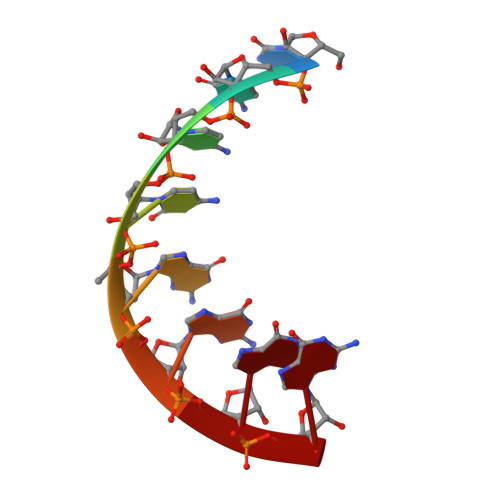
6VEM - PubMed Abstract:
Various chemical modifications have been identified that enhance potency of small interfering RNAs (siRNAs) and that reduce off-target effects, immune stimulation, and toxicities of metabolites of these therapeutic agents. We previously described 5'-C-methyl pyrimidine nucleotides also modified at the 2' position of the sugar. Here, we describe the synthesis of 2'-position unmodified 5'-(R)- and 5'-(S)-C-methyl guanosine and evaluation of these nucleotides in the context of siRNA. The (R) isomer provided protection from 5' exonuclease and the (S) isomer provided protection from 3' exonuclease in the context of a terminally modified oligonucleotide. siRNA potency was maintained when these modifications were incorporated at the tested positions of sense and antisense strands. Moreover, the corresponding 5' triphosphates were not substrates for mitochondrial DNA polymerase. Models generated based on crystal structures of 5' and 3' exonuclease oligonucleotide complexes with 5'-(R)- and 5'-(S)-C-methyl substituents attached to the 5'- and 3'-terminal nucleotides, respectively, provided insight into the origins of the observed protections. Structural properties of 5'-(R)-C-methyl guanosine incorporated into an RNA octamer were analysed by X-ray crystallography, and the structure explains the loss in duplex thermal stability for the (R) isomer compared with the (S) isomer. Finally, the effect of 5'-C-methylation on endoribonuclease activity has been explained.
Organizational Affiliation:
Alnylam Pharmaceuticals, 675 West Kendall Street, Cambridge, Massachusetts 02142, USA.