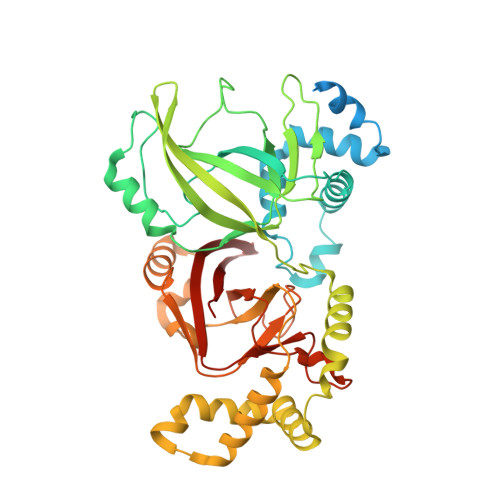
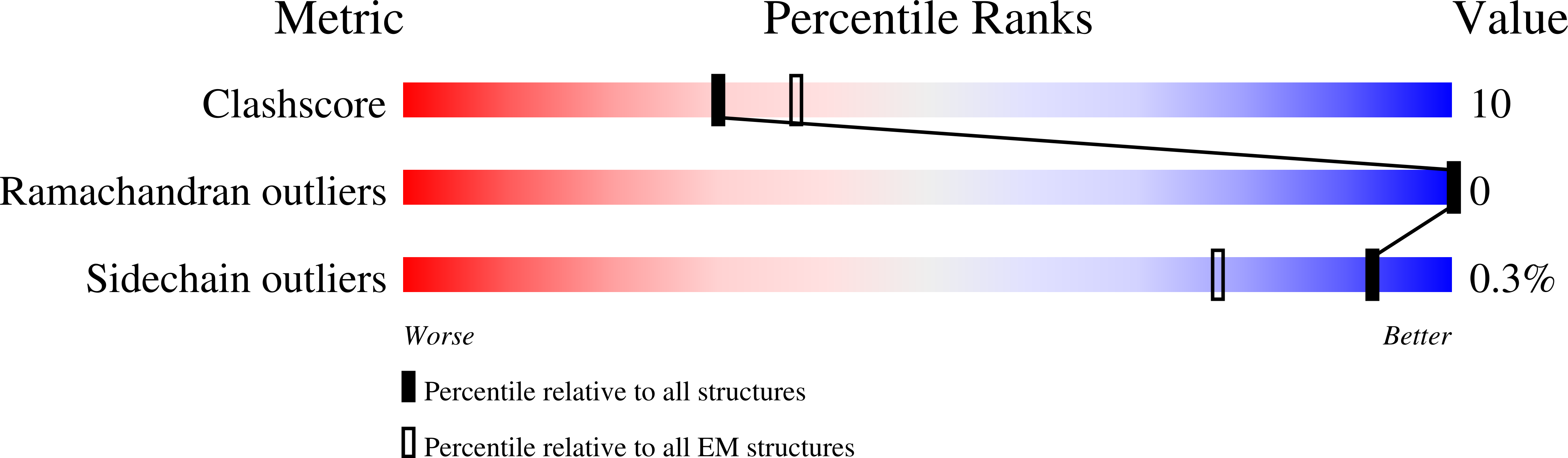Structural elucidation of theClostridioides difficiletransferase toxin reveals a single-site binding mode for the enzyme.
Sheedlo, M.J., Anderson, D.M., Thomas, A.K., Lacy, D.B.(2020) Proc Natl Acad Sci U S A 117: 6139-6144
- PubMed: 32123082
- DOI: https://doi.org/10.1073/pnas.1920555117
- Primary Citation of Related Structures:
6V1S - PubMed Abstract:
Clostridioides difficile is a Gram-positive, pathogenic bacterium and a prominent cause of hospital-acquired diarrhea in the United States. The symptoms of C. difficile infection are caused by the activity of three large toxins known as toxin A (TcdA), toxin B (TcdB), and the C. difficile transferase toxin (CDT). Reported here is a 3.8-Å cryo-electron microscopy (cryo-EM) structure of CDT, a bipartite toxin comprised of the proteins CDTa and CDTb. We observe a single molecule of CDTa bound to a CDTb heptamer. The formation of the CDT complex relies on the interaction of an N-terminal adaptor and pseudoenzyme domain of CDTa with six subunits of the CDTb heptamer. CDTb is observed in a preinsertion state, a conformation observed in the transition of prepore to β-barrel pore, although we also observe a single bound CDTa in the prepore and β-barrel conformations of CDTb. The binding interaction appears to prime CDTa for translocation as the adaptor subdomain enters the lumen of the preinsertion state channel. These structural observations advance the understanding of how a single protein, CDTb, can mediate the delivery of a large enzyme, CDTa, into the cytosol of mammalian cells.
Organizational Affiliation:
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, TN 37232.