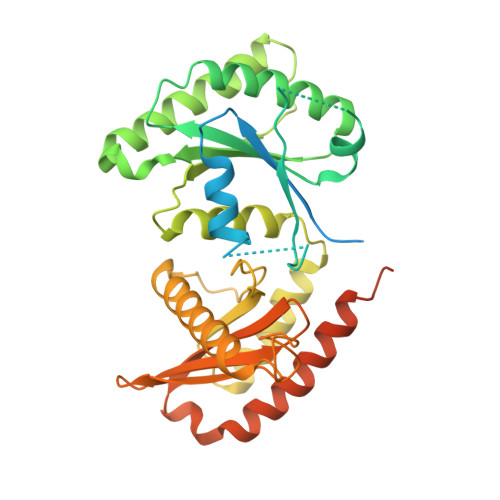
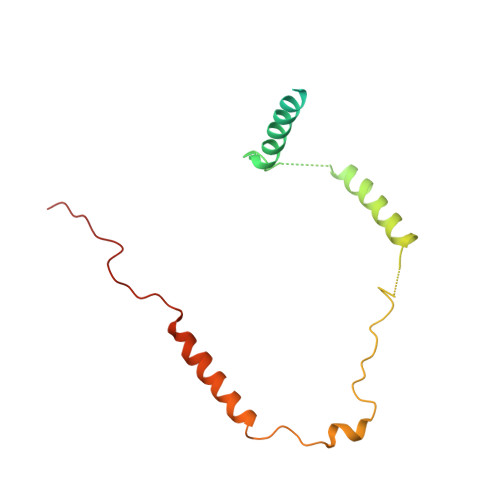
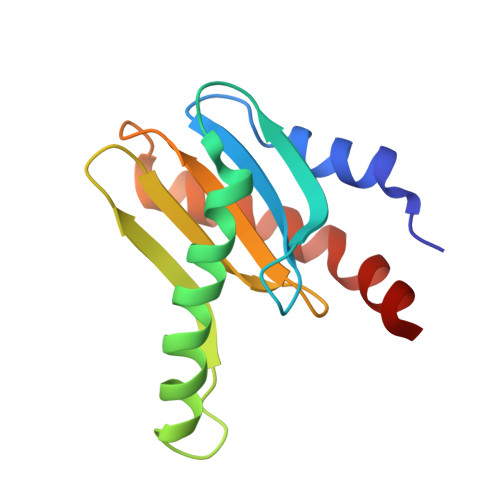
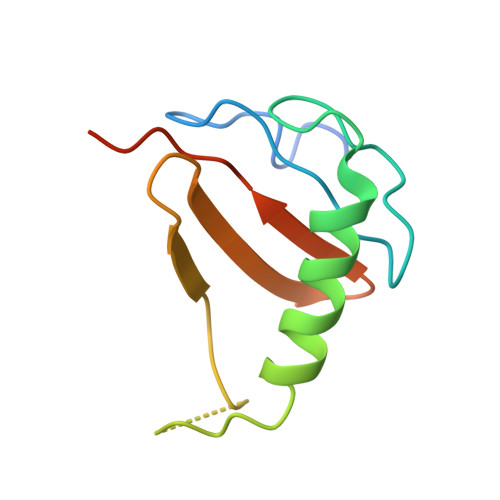
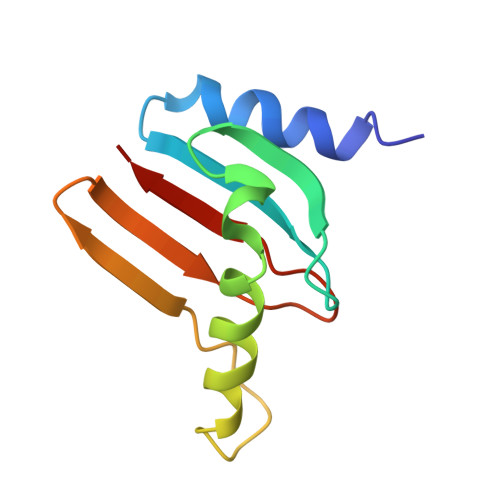
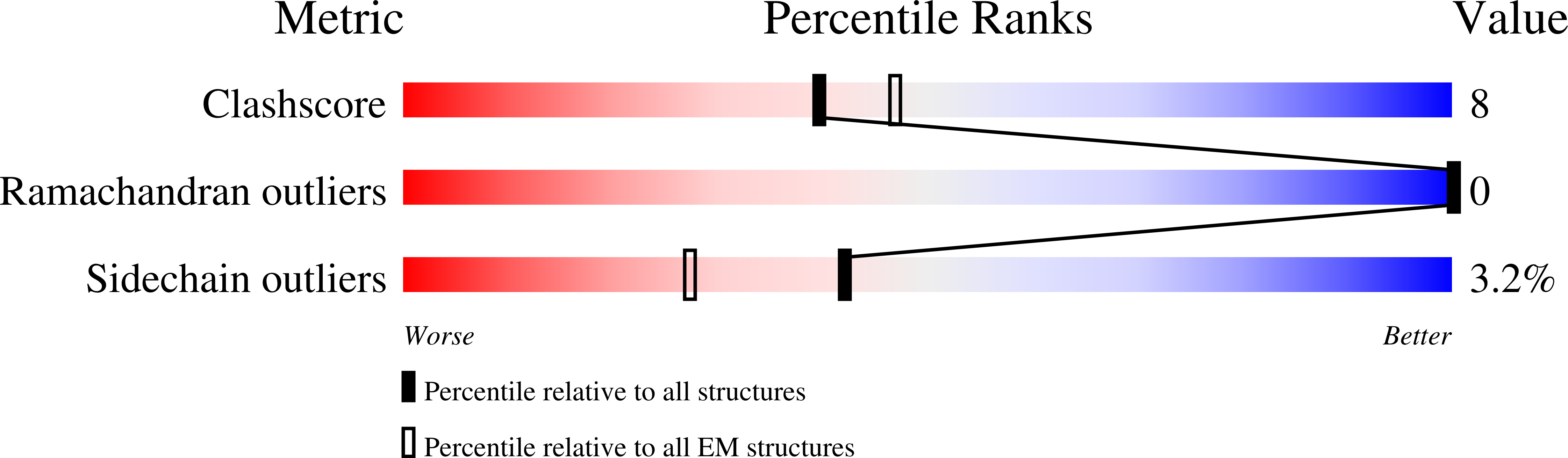Structural basis for the docking of mTORC1 on the lysosomal surface.
Rogala, K.B., Gu, X., Kedir, J.F., Abu-Remaileh, M., Bianchi, L.F., Bottino, A.M.S., Dueholm, R., Niehaus, A., Overwijn, D., Fils, A.P., Zhou, S.X., Leary, D., Laqtom, N.N., Brignole, E.J., Sabatini, D.M.(2019) Science 366: 468-475
- PubMed: 31601708
- DOI: https://doi.org/10.1126/science.aay0166
- Primary Citation of Related Structures:
6U62 - PubMed Abstract:
The mTORC1 (mechanistic target of rapamycin complex 1) protein kinase regulates growth in response to nutrients and growth factors. Nutrients promote its translocation to the lysosomal surface, where its Raptor subunit interacts with the Rag guanosine triphosphatase (GTPase)-Ragulator complex. Nutrients switch the heterodimeric Rag GTPases among four different nucleotide-binding states, only one of which (RagA/B•GTP-RagC/D•GDP) permits mTORC1 association. We used cryo-electron microscopy to determine the structure of the supercomplex of Raptor with Rag-Ragulator at a resolution of 3.2 angstroms. Our findings indicate that the Raptor α-solenoid directly detects the nucleotide state of RagA while the Raptor "claw" threads between the GTPase domains to detect that of RagC. Mutations that disrupted Rag-Raptor binding inhibited mTORC1 lysosomal localization and signaling. By comparison with a structure of mTORC1 bound to its activator Rheb, we developed a model of active mTORC1 docked on the lysosome.
Organizational Affiliation:
Whitehead Institute for Biomedical Research, Cambridge, MA 02142, USA.